-
PDF
- Split View
-
Views
-
Cite
Cite
Judah Goldstein, Ruth E. Hubbard, Paige Moorhouse, Melissa K. Andrew, Arnold Mitnitski, Kenneth Rockwood, The validation of a care partner-derived frailty index based upon comprehensive geriatric assessment (CP-FI-CGA) in emergency medical services and geriatric ambulatory care, Age and Ageing, Volume 44, Issue 2, March 2015, Pages 327–330, https://doi.org/10.1093/ageing/afu161
Close - Share Icon Share
Abstract
Background: the derivation of a frailty index (FI) based on deficit accumulation from a Comprehensive Geriatric Assessment (CGA) has been criticised as cumbersome. To improve feasibility, we developed a questionnaire based on a CGA that can be completed by care partners (CP-FI-CGA) and assessed its validity.
Methods: we enrolled a convenience sample of patients aged 70 or older (n = 203) presenting to emergency medical services (EMS) or geriatric ambulatory care (GAC). To test construct validity, we evaluated the shape of the CP-FI-CGA distribution, including its maximum value, relationship with age and gender. Criterion validity was evaluated by survival analysis and by the correlation between the CP-FI-CGA and specialist-completed FI-CGA.
Results: the mean age was 82.2 ± 5.9 years. Most patients were women (62.1%), unmarried (widowed, divorced and single) (59.6%) and lived in their own home or apartment (78.3%). The mean CP-FI-CGA was 0.41 ± 0.15 and was higher in the EMS group (0.45 ± 0.15) than in GAC (0.37 ± 0.14) (P < 0.001). The CP-FI-CGA correlated well with the specialist-completed FI-CGA (0.7; P < 0.05). People who died had a higher CP-FI-CGA than did survivors (0.48 ± 0.13 versus 0.38 ± 0.15). Each 0.01 increase in the FI was associated with a higher risk of death (HR 1.04; 95% CI 1.02–1.06).
Conclusion: the CP-FI-CGA has properties that resemble other published FIs and may be useful in busy clinical practice for grading degrees of frailty. It efficiently integrates information from care partners so that it can help guide decision-making.
Introduction
Frailty is a state of vulnerability and susceptibility to adverse health outcomes [1]. How best to measure frailty in clinical practice is disputed [2, 3], even recognising that differing settings will likely require different approaches [4]. Quick, easily applied measures that capture risk and can be interpreted accurately during an acute illness are needed. This is true in acute care, including emergency medical services (EMS), which are used frequently by frail older adults [5, 6].
The frailty index (FI) measures the degree of deficit accumulation [7]. As people age, they accumulate health problems that give rise to frailty [8]. The FI has characteristic properties, including a sub-maximal value near 0.7 [8–10]. The FI is multidimensional, typically including 30 or more health variables [11]. Even so, and despite widespread use of electronic health records that contain large volumes of information, the FI has been criticised as being too cumbersome for clinical care [12]. Comprehensive Geriatric Assessment (CGA) captures health information so that issues can be addressed systematically [13, 14], and can be used to calculate an FI (FI-CGA) [15–18]. Most of this information is known to care partners.
Our objectives were to explore the validity of a care partner-derived FI using a questionnaire based upon a CGA (CP-FI-CGA). We evaluated its validity (content, construct and criterion) in two busy environments: EMS and geriatric ambulatory care (GAC).
Methods
Study design, setting and population
Participants aged 70+ were enrolled in two settings. The sample size (target 200) was powered on a correlation analysis, with an expected correlation of 0.8 (± 0.2) between the FI-CGA and CP-FI-CGA [19]. Subjects had to be accompanied by a knowledgeable care partner. The survey was conducted in English. Exclusion criteria were inability to complete the questionnaire or refusal to participate. Additional details about methods unique to each setting have been reported elsewhere [20, 21]. CGA is a part of routine care in GAC. The inclusion of EMS enabled assessment in an especially time-sensitive clinical environment. The study was approved by the Capital Health research ethics committee (CDHA-RS/2009-138).
Data collection
The 62-question CP-CGA [20] was based on an in-hospital CGA [8]. Recruitment was at the discretion of the attending health-care provider. Refusals and number of eligible patients are unknown, as care partner presence is not routinely tracked. The care partner completed the CP-CGA while the patient was being assessed and treated. Follow-up occurred using a structured data collection form after 1 year.
Frailty measures
Construction of the CP-FI-CGA followed a standard procedure [11]. Briefly, the FI is the proportion of deficits present in an individual out of a possible 44 items (Supplementary data, Appendix 1 available in Age and Ageing online). (The remaining 18 questions covered demographic and social information and were not included in the FI.) An FI was not calculated if <60% of the CP-CGA was completed. The care partner described the patient's state 2 weeks prior to the current encounter (baseline FI) and during their current encounter (current FI). Responses were categorised as ‘yes’ or ‘no’ with some intermediate values.
Data analysis
Construct validation was performed by comparing frailty measures with each other and with measures of relevant patient characteristics (e.g. activities of daily living, age and cognition). Frailty was also compared with the Canadian Triage and Acuity Scale (CTAS), a surrogate marker of illness severity. Criterion validity was assessed by evaluating Kaplan–Meier curves, and Cox regression adjusted for age, gender, and setting (EMS = 1, GAC = 2).
Results
Of 203 subjects enrolled, 5 were withdrawn for not meeting the eligibility criteria and 5 due to missing baseline data (<60%). One-year follow-up was not possible for an additional 12 subjects. Patients were older (82.2 ± 5.9 years), mostly women (62.1%) and lived in their own home or apartment (78.3%). In both settings, the care partner typically was an offspring.
The mean CP-FI-CGA at baseline was 0.39 ± 0.15 and 0.41 ± 0.15 at the current encounter (P < 0.001). The mean CP-FI-CGA (current) was higher in the EMS group (0.45 versus 0.36; P < 0.001). The specialist-derived FI-CGA and the CP-FI-CGA were moderately correlated (r = 0.7, P < 0.05). The CP-FI-CGA was normally distributed around the high mean (0.41) with a maximum observed value of 0.73.
The FI correlated with age r = 0.2 (P < 0.05). There was no difference in the mean FI for women (0.42 ± 0.15) compared with men (0.40 ± 0.15; P = 0.52). Those in the severely frail group (>0.5) were slightly older, women and had multiple co-morbidities, problems in balance and cognition (Table 1). The CP-FI-CGA correlated with disability but not cognition (Supplementary data, Appendix 2 available in Age and Ageing online). There was no association between the CTAS and frailty, however defined.
Characteristics of EMS and GAC patients in relation to current frailty status
| CP-FI-CGA . | CP-FI-CGA group . | ||
|---|---|---|---|
| <0.3 . | 0.3–0.5 . | >0.5 . | |
| n | 47 | 77 | 57 |
| Mean age (SD) | 80.2 (5.3) | 82.3 (5.5) | 83.5 (6.5) |
| % of women | 61.7 | 58.7 | 65.5 |
| % with memory problems | 57.8 | 70.5 | 75 |
| % with falls | 22.4 | 56.8 | 76.3 |
| Mean number of co-morbidities (SD) | 2.3 (1.6) | 3.9 (1.7) | 5.54 (1.8) |
| % with five or more medications | 58.3 | 73.1 | 86.2 |
| % classified as CTAS III | 72.2 | 77.8 | 75.6 |
| ED LOS (h), mean (SD) | 12.8 (11.3) | 18.2 (11.4) | 14.5 (11.2) |
| In-hospital CGA | 60 | 54.8 | 40.7 |
| CP-FI-CGA . | CP-FI-CGA group . | ||
|---|---|---|---|
| <0.3 . | 0.3–0.5 . | >0.5 . | |
| n | 47 | 77 | 57 |
| Mean age (SD) | 80.2 (5.3) | 82.3 (5.5) | 83.5 (6.5) |
| % of women | 61.7 | 58.7 | 65.5 |
| % with memory problems | 57.8 | 70.5 | 75 |
| % with falls | 22.4 | 56.8 | 76.3 |
| Mean number of co-morbidities (SD) | 2.3 (1.6) | 3.9 (1.7) | 5.54 (1.8) |
| % with five or more medications | 58.3 | 73.1 | 86.2 |
| % classified as CTAS III | 72.2 | 77.8 | 75.6 |
| ED LOS (h), mean (SD) | 12.8 (11.3) | 18.2 (11.4) | 14.5 (11.2) |
| In-hospital CGA | 60 | 54.8 | 40.7 |
The CP-FI-CGA was not calculated where the completeness of items was <60% (n = 5). Matching the CP-FI-CGA to in-hospital records was not possible for an additional 12 subjects.
CTAS, Canadian Triage and Acuity Scale; N/A, not applicable; CP-FI-CGA, care partner-completed frailty index; FI-CGA, specialist-completed frailty index; ED LOS, emergency department length of stay.
Characteristics of EMS and GAC patients in relation to current frailty status
| CP-FI-CGA . | CP-FI-CGA group . | ||
|---|---|---|---|
| <0.3 . | 0.3–0.5 . | >0.5 . | |
| n | 47 | 77 | 57 |
| Mean age (SD) | 80.2 (5.3) | 82.3 (5.5) | 83.5 (6.5) |
| % of women | 61.7 | 58.7 | 65.5 |
| % with memory problems | 57.8 | 70.5 | 75 |
| % with falls | 22.4 | 56.8 | 76.3 |
| Mean number of co-morbidities (SD) | 2.3 (1.6) | 3.9 (1.7) | 5.54 (1.8) |
| % with five or more medications | 58.3 | 73.1 | 86.2 |
| % classified as CTAS III | 72.2 | 77.8 | 75.6 |
| ED LOS (h), mean (SD) | 12.8 (11.3) | 18.2 (11.4) | 14.5 (11.2) |
| In-hospital CGA | 60 | 54.8 | 40.7 |
| CP-FI-CGA . | CP-FI-CGA group . | ||
|---|---|---|---|
| <0.3 . | 0.3–0.5 . | >0.5 . | |
| n | 47 | 77 | 57 |
| Mean age (SD) | 80.2 (5.3) | 82.3 (5.5) | 83.5 (6.5) |
| % of women | 61.7 | 58.7 | 65.5 |
| % with memory problems | 57.8 | 70.5 | 75 |
| % with falls | 22.4 | 56.8 | 76.3 |
| Mean number of co-morbidities (SD) | 2.3 (1.6) | 3.9 (1.7) | 5.54 (1.8) |
| % with five or more medications | 58.3 | 73.1 | 86.2 |
| % classified as CTAS III | 72.2 | 77.8 | 75.6 |
| ED LOS (h), mean (SD) | 12.8 (11.3) | 18.2 (11.4) | 14.5 (11.2) |
| In-hospital CGA | 60 | 54.8 | 40.7 |
The CP-FI-CGA was not calculated where the completeness of items was <60% (n = 5). Matching the CP-FI-CGA to in-hospital records was not possible for an additional 12 subjects.
CTAS, Canadian Triage and Acuity Scale; N/A, not applicable; CP-FI-CGA, care partner-completed frailty index; FI-CGA, specialist-completed frailty index; ED LOS, emergency department length of stay.
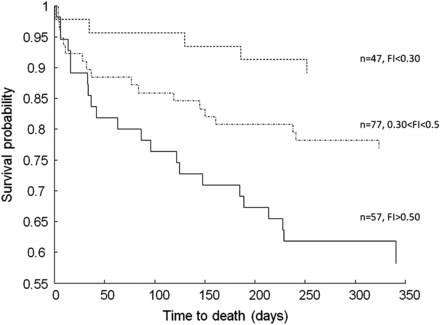
The 1-year mortality rate was 23.7% (47/198) (31.7% EMS versus 15.4% GAC). A non-significant trend towards an increasing death rate with age was observed. The relative risk (RR) of death accelerated with increasing frailty and was 2.15 (95% CI 0.86–5.4) for those with a FI from 0.3 to 0.5 and 3.87 (95% CI 1.6–9.35) for those with a FI > 0.5. The CP-FI-CGA stratified survival over 1 year with worse survival in the highest levels of frailty (Figure 1 ). The current CP-FI-CGA strongly predicted survival: HR of 1.04 (1.02–1.06) adjusting for age (HR 1.02, 95% CI 0.97–1.07), setting (1.63, 95% CI 0.86–3.1) and gender (2.78, 95% CI 1.54–5.02) for each 1% increment. For example, compared with a 70-year-old woman with a CP-FI-CGA score of 0.10, an 80-year-old man with an CP-FI-CGA score of 0.50 would have a risk of death of 2.82, with the hazard calculated as follows: age-associated risk [ln (1.02)*10] and being male [ln (2.78)] and FI-associated RR of death [ln (1.04)*40]. The specialist-completed FI-CGA (current) remained the better predictor of survival (HR 1.05, 95% CI 1–1.1; AUC 0.714, 95% CI 0.586–0.842) compared with the care partner CP-FI-CGA (0.706, 95% CI 0.622–0.79).
Kaplan–Meier survival curves stratified by CP-FI-CGA score. The number of people in each group is indicated to the right of the curve (n = 181).
Discussion
We developed a questionnaire (CP-CGA) that facilitates frailty assessment during the course of clinical care by capturing the knowledge of care partners. Carers contribute a wealth of knowledge, most notably in the presence of cognitive impairment, that can be summarised in an FI (CP-FI-CGA). Prior FIs have relied on self-reported data, clinical assessments or performance-based measures. In feasibility studies, we demonstrated that it took about 15 min to complete the CP-CGA [20, 21]. This is the first reported use of the FI using care partner-reported information and in an EMS population.
The high mean CP-FI-CGA (0.41) indicates a high burden of frailty and is comparable with other FI-CGAs [16, 18, 22, 23]. Similarly, the normal distribution reported here is consistent with the FI becoming less skewed at advanced ages and normally distributed in clinical samples [7, 24, 25]. Note also that, as in other clinical reports, most patients (n = 169; 88%) had FIs > 0.22, a common cut point when the FI is dichotomised, showing the merit of distinguishing grades of frailty [26].
The moderate correlation (0.7) between the CP-FI-CGA and specialist-completed FI-CGA, despite similar predictive validity, likely reflects the settings, but varying differences in care partners reporting deficits versus clinical judgment, and measurement error cannot be excluded. Furthermore, only 26 in-hospital CGAs were performed on EMS patients, and some deficits may have changed during the course of care which would not be the case for GAC. The FI-CGA was completed less often in the most severely frail. This may be due to these patients occasionally attending the emergency department for specific issues (e.g. catheter problem).
Our data must be interpreted with caution. Enrolment was at the discretion of the health-care provider. Most EMS patients were enrolled during off-load delays where paramedics maintained care. There were no CTAS 1 (highest acuity) patients, reflecting current practice, which emphasises immediate transport. Even so, the great majority of people aged 70 and older are CTAS 2 or higher [27], and currently paramedics spend time with them, allowing collection of data on pre-morbid function. In as much as it is difficult, in the course of current hospital stays, to make people better than they were 2 weeks before they came to hospital, knowing this information more systematically through information gathering could aid in care planning [28]. The small sample size and enrolment process impair generalisability. Refusals and eligibility criteria were not tracked. A larger, multi-site trial is warranted. Finally, acute severity of illness bias may have contributed to the higher FIs noted in the pre-hospital setting. Even so, care partner stress was similar between groups.
Frailty viewed as deficit accumulation has some strengths. The FI can grade degrees of frailty which can aid in understanding prognosis and in establishing goals of care. Using care partner-reported information addresses the criticism that the FI might be too cumbersome. The management of older adults in emergency medicine is of concern with adverse events associated with prolonged stays [29] and the risk of under-triage of those with non-specific complaints [30]. Frailty assessment may help overcome these dilemmas.
Conclusion
The CP-FI-CGA gathers much of the information necessary for frailty quantification from care partners. Whether this information can improve care is motivating additional inquiries by our group.
• Care partners can provide enough detailed information to contribute to a frailty assessment.
• A care partner-derived FI is a strong predictor of the risk of death in emergency services and outpatient clinics.
• The Comprehensive Geriatric Assessment (CP-FI-CGA) may be useful in busy clinical practice for grading degrees of frailty.
Conflicts of interest
None declared.




Comments