-
PDF
- Split View
-
Views
-
Cite
Cite
Chris Moyses, Alex Hearn, Andrew Redfern, Evaluation of a Novel Nicotine Inhaler Device: Part 2—Effect on Craving and Smoking Urges, Nicotine & Tobacco Research, Volume 17, Issue 1, January 2015, Pages 26–33, https://doi.org/10.1093/ntr/ntu122
Close - Share Icon Share
Abstract
Many smokers find currently available nicotine replacement therapies unsatisfactory. The pharmacokinetics of nicotine delivered via a novel inhaler device, and its effect on craving satiation and smoking urges, were compared with the Nicorette® Inhalator (10mg).
Results are reported for Parts B (N = 24) and D (N = 24) of a 4-part Phase I study. Participants (18–55 years, ≥10 cigarettes/day within 1hr of waking, expired carbon monoxide >10 ppm on screening) received single doses of nicotine on consecutive days (0.45 and 0.67mg [Part B] and 0.45mg [Part D] via the novel device; 10mg via Nicorette® [Parts B and D]). Venous pharmacokinetics, craving, and tolerability were assessed.
In Part B, the novel device 0.45 and 0.67mg produced significantly lower Cmax, AUClast, and AUCall than Nicorette® (all p ≤ .05), higher AUC0–10 and significantly shorter Tmax (18.7 and 19.2min vs. 38.0min, respectively, p ≤ .05). Craving score AUC was lower for the novel device 0.45mg than for Nicorette® in Part B (1356.3 vs. 1566.3, p = .029) and approached statistical significance in Part D (1208.5 vs. 1402.3 [p = .059]). Mean craving scores were lower for the novel device 0.45mg than Nicorette® at 7/8 postdose timepoints in Part B (p ≤ .05 at 180 and 240min) and at all timepoints in Part D (p ≤ .05 at 2, 4, and 10min).
The novel device was at least as effective as the Nicorette® Inhalator (10mg) in relieving craving and smoking urges and was statistically superior at certain timepoints and in an overall craving AUC analysis, despite lower total nicotine exposure.
Introduction
Tobacco remains the single greatest preventable cause of morbidity and premature death worldwide.1 Nicotine replacement therapy (NRT) is the most widely used treatment for tobacco dependence2 and is available in a variety of presentations. However, the improvements in smoking cessation rates that are achieved by current products are modest. In the United Kingdom, the proportion of smokers who successfully quit for 52 weeks without any behavioral support using NRT products in primary and secondary care is estimated as just 7%–10%.3
Smokers may fail to quit because the currently available NRTs do not deliver nicotine in the same way as cigarettes,4,5 with slower onset of systemic nicotine delivery. Furthermore, most of the existing NRTs do not replace the unique sensory cues or rituals of smoking.6,7 More effective and acceptable forms of NRT are needed to help smokers quit and to provide a satisfactory alternative to smoking for those unable to stop.8 In the United Kingdom, recently published recommendations advocate the use of licensed nicotine-containing products to help people cut down before stopping smoking, to reduce their smoking frequency, or to abstain from smoking using a harm-reduction approach.8,9
A new inhaler has been developed that delivers a nicotine aerosol and replicates many of the rituals associated with smoking. The device is the size and shape of a conventional cigarette and comprises a small, breath-operated valve that allows a user to self-titrate the inhalation. The rate at which nicotine is delivered from one charge of the inhaler device is therefore dependent on the number of “puffs” over which one charge (or “dose”) is inhaled and this, in turn, depends on an individual’s depth of inhalation. For most users, the device provides a comparable number of puffs to that of a conventional tobacco cigarette. The device can be refilled approximately 20 times via a pressurized canister of pharmaceutical grade (free-base) nicotine formulated with propylene glycol, ethanol, saccharin, menthol, and HFA134a (chlorofluorocarbon free) propellant. The canister forms an integral part of the storage container, which is similar in size to a pack of 20 cigarettes. The novel nicotine inhaler is shown in Supplementary Figure S1. The novel nicotine delivery device contains no tobacco and requires neither combustion nor heat in its operation. This contrasts to electronic cigarettes where heat is required to vaporize the nicotine. Furthermore, electronic cigarette brands and models differ in their efficacy and consistency of nicotine vaporization10 and few have undergone pharmacokinetic and safety analyses or formal assessment of their effectiveness on craving.11,12 The amount of nicotine inhaled from 15 puffs of an electronic cigarette that vaporizes nicotine effectively is far lower than from smoking a conventional cigarette.13
It is important that any new NRT relieves symptoms of tobacco withdrawal and the craving or desire to smoke. The relief of such symptoms is thought to mediate the clinical effect of abstinence, and their measurement can be used to assess the effectiveness of a nicotine replacement agent and to predict quit success.14
We performed a four-part study to evaluate nicotine delivery from this novel device. The two parts reported here were similarly designed to compare the pharmacokinetics (PK) of nicotine delivery from this novel device and the Nicorette® Inhalator (10mg), and their effectiveness on craving satiation and smoking urges. The results of the remaining two study parts are to be discussed separately (CM, AH, AR, in progress).
Methods
Study Design
This Phase I study comprised four parts: A, B, C, and D:
Part A: A randomized, single-blind, multidose study to determine the arterial PK of orally inhaled nicotine via the novel nicotine inhaler device at three nicotine dose levels: 0.22, 0.45, and 0.67mg (Moyses, Hearn, and Redfern,15 poster presented at Society for Research on Nicotine and Tobacco).
Part B: A randomized, open-label, single-blind, three-way crossover study to determine the venous PK of orally inhaled nicotine at two dose levels (0.45 and 0.67mg) delivered via the novel nicotine inhaler device compared with the Nicorette® Inhalator (10mg).
Part C: An open-label study to determine the tolerability and venous PK of repeat doses of orally inhaled nicotine delivered via the novel nicotine inhaler device at one dose level (0.67mg).
Part D: A randomized, open-label, two-way crossover study to determine the venous PK of orally inhaled nicotine at one dose level (0.45mg) delivered via the novel nicotine inhaler device compared with the Nicorette® Inhalator (10mg).
The study (Australian New Zealand Clinical Trials Registry Number 343206) was approved by the Queensland Clinical Trials Network Inc. (Human Research Ethics Committee Number 2011003) in accordance with the International Conference on Harmonization Good Clinical Practice guidelines. The study was conducted at a single center in Perth, Australia, between January 9, 2012, and July 6, 2012, and all participants provided written informed consent prior to study start.
Participants
Healthy volunteers (male or female) aged 18–55 years were eligible to participate if they had smoked at least 10 manufactured cigarettes per day for the last year and smoked their first cigarette within 1hr of waking. Women of childbearing potential were eligible only if they tested negative for pregnancy prior to study start and were using an accepted method of contraception. All participants had an expired carbon monoxide level of at least 10 ppm at screening and were required to abstain from smoking for 12hr prior to their scheduled dosing time.
Participants were excluded if they had a known or suspected history of hypersensitivity to nicotine or any other component of the inhaler or Nicorette® Inhalator. Participants were also excluded if they had a history of confirmed chronic and/or serious pulmonary disease, including asthma, or chronic obstructive pulmonary disease, a history of myocardial infarction or cerebrovascular accident, other clinically significant cardiac or renal conditions, or any comorbidity that could place them at risk or interfere with the interpretation of the study data. Pregnant or breast-feeding women were excluded from the study.
Study Treatment
Participants were familiarized with the use of the novel device using a placebo formulation on the day prior to receiving active treatment. The placebo formulation was identical to the active formulation with the exception of nicotine. Study participants were blinded to the dose of nicotine administered in each part of the study. Participants inhaled the contents of one charge of the novel device in a similar way to a cigarette. All participants were instructed to inhale the dose at the same rate of one inhalation every 15 s over approximately 2min (i.e., eight inhalations in total). The fine particle dose (<5 µm) has a specification of 160–305 µg for a formulation concentration of 0.056% wt/wt nicotine (i.e., 35%–68% of the nicotine dose). The nicotine dose contained in 0.8g of formulation (a single dose/refill of the novel device) is estimated to be 0.45 and 0.67mg for the 0.056% wt/wt and 0.084% wt/wt concentrations, respectively. The Nicorette® Inhalator (10mg) is an orally inhaled NRT product and was selected as the comparator because it was the closest available presentation to the novel inhaler device. Participants took one inhalation of the Nicorette® Inhalator (10mg) every 15 s, taking no longer than 20min (i.e., 80 inhalations) to complete the dose, in line with the regimen described in the prescribing information.16 The available nicotine dose per cartridge of Nicorette® Inhalator (10mg) is estimated to be 4mg but is temperature dependent.16 The time at which participants started to take the first inhalation of the test product was recorded as the dose time (t = 0).
In Part B, each participant received a single dose of nicotine on three consecutive days, at dose levels of 0.45 and 0.67mg via the novel nicotine inhaler device, and a single dose via the Nicorette® Inhalator (10mg).
In Part D, each participant received a single dose of nicotine via the novel nicotine inhaler device (0.45mg) and a single dose of nicotine via the Nicorette® Inhalator (10mg) on two consecutive days. For the novel device, Part B tested the first refill, whereas Part D examined the fourth refill. During the early stages of the study, it was noted that the quantity of nicotine delivered via the novel inhaler device for the first dose (i.e., one complete refill) tended to be only approximately 70% that of subsequent doses. In Part D of the study, the fourth refill of the novel device was investigated by filling and flushing the device three times, using a pump (Cole-Parmer), a Critical Flow Controller (Model TPK), and the Dose Uniformity Sampling Apparatus. Administration of the Nicorette® Inhalator (10mg) was identical in both study parts.
Study Assessments
PK Analysis
PK assessment was the primary outcome measure for this study. For both Parts B and D, venous blood samples were collected 5min (±1min) predose and at 2, 4, 7, and 10min (±1min), 15, 20, 30, 40, 50, and 60min (±2min), and 120, 180, 240, and 300min (±5min) postdose (i.e., from the start of inhalation) for the measurement of plasma nicotine concentration by a liquid chromatography with tandem mass spectrometry method. The method was validated for linearity, precision, and accuracy. Quality control samples at concentrations of 3.0, 7.5, and 37.5ng/ml, as well as 37.5ng/ml used as dilute quality control for samples of low volume (diluted 1 in 2), were used to determine inter- and intraprecision and inter- and intra-accuracy. The mean inter-run accuracy was within 1% and precision was within 5%. Derived PK parameters were summarized separately by device and dose level.
Efficacy
Efficacy was evaluated by assessing the impact of the test devices on craving satiation and smoking urges, and their effect on aspects of nicotine withdrawal, using
A Visual Analog Scale (VAS) to assess the level of craving of the subject based on their response to the question, “How strong is your craving for cigarettes?” on a scale of 0 (no craving) to 10 (strong craving). This assessment was made predose and at 4, 20, 40, 60, 120, 180, 240, and 300min from the start of dosing. In Part D, additional assessments were made at 2 and 10min from the start of dosing.
The Questionnaire on Smoking Urges (QSU-Brief)17 to assess the level of craving and smoking urges based on the responses of participants to 10 statements on a scale of 1 (strongly disagree) to 7 (strongly agree). These assessments were made predose and at 20, 40, 60, 120, 180, 240, and 300min from the start of dosing. Component scores were determined for the “desire” and “anticipation” subscales. Results of the “anticipation” component score of the QSU-Brief were used as a measure of nicotine craving.
Safety
Safety and tolerability of the novel device and Nicorette® Inhalator were compared. Local tolerability was an assessment of the contact area of the novel device or Nicorette® Inhalator with the participant’s lips. Participants were asked how the device felt in their mouth or lips, which were also assessed visually. Tolerability was an assessment of symptoms resulting from the oral inhalation from the inhalers. Any symptoms that were reported as worse than prior to dosing were recorded as adverse events (AEs). AEs were ascertained by neutral questioning and their incidence and nature were recorded and also rated by the investigator as “not related,” “possibly related,” “probably related,” and “definitely related” to the test product. Physical examination and monitoring of vital signs (blood pressure, heart rate, respiration rate, and temperature) were also conducted.
For both Parts B and D, assessments of local tolerability and vital signs were performed at 20min predose. Postdose assessments measured after the start of dosing comprised local tolerability at 4 and 20min; physical examination at 300min; and vital signs and AEs, SAEs, and concomitant medicine at 4, 20, 40, 60, 120, 180, 240, and 300min. All participants were contacted by telephone (9±2 days) following the study end and any AEs, SAEs, and concomitant medicines were recorded. Treatment-emergent AEs (TEAEs) were evaluated from the start of dosing of the test product until the safety telephone follow-up call.
Statistical Analyses
Participants were included in the PK population if they received all of the planned doses of nicotine. Participants were included in the safety intent-to-treat population if they received one dose of nicotine. Statistical analyses were conducted using SAS® Version 9.2.
PK Analysis
Plasma concentrations of nicotine were measured over time and derived PK parameters were summarized by device and dose level separately for both Parts B and D of the study. The PK parameters determined were the mean maximum plasma nicotine concentration (Cmax), the mean time to maximum plasma nicotine concentration (Tmax), and the mean area under the plasma concentration–time curve (AUC), from time zero to the end of the sample collection period (AUCall) and from time zero to the time of the last quantifiable concentration (AUClast), following administration of nicotine using either device. Comparative analysis between both dose levels of the novel nicotine inhaler device and the Nicorette® Inhalator (10mg) was performed using an analysis of variance (ANOVA) with logarithmic transformation of Cmax and AUC values. Differences of p ≤ .05 were considered significant.
PK analysis was conducted using Phoenix™ WinNonlin® Version 6.2.
Efficacy
Craving measures and the QSU-Brief total and component scores were summarized over time by device and dose level in both study Parts B and D. A paired Student’s t test was used to analyze the difference in mean craving VAS and QSU-Brief scores by device and timepoint. Craving was also assessed from the area under the VAS score–time curve, where a lower craving AUC equates to a better craving reduction. Comparative analysis between both dose levels of the novel nicotine inhaler device and the Nicorette® Inhalator (10mg) of efficacy was performed using an ANOVA with logarithmic transformation of AUC.
The number of participants in Parts B and D was sufficient to demonstrate equivalence at a similarity margin of 20% with 80% power using the two one-sided 5% tests approach, assuming an underlying intrasubject coefficient of variation of 15%.
The quantity of nicotine inhaled from the novel device by each participant was calculated from the weight of formulation emitted by the device and the target nicotine concentration of the formulation.
Results
Study Population
A total of 24 participants were randomized for Part B and a further 24 for Part D of the study. Mean ages of participants were 28.6 years (Part B) and 29.7 years (Part D), and 58% (Part B) and 54% (Part D) were male.
Part B
All 24 participants in Part B received a single dose from the Nicorette® Inhalator (10mg), but only 23 received a single dose of each of two dose levels of the novel nicotine inhaler device as one participant withdrew because of study restrictions. The mean (SD) weights of formulation inhaled from the novel device were 0.9472g (0.2051g) and 0.8610g (0.3005g), corresponding to 0.5304 and 0.7232mg nicotine from the novel device 0.45 and 0.67mg, respectively. There were no withdrawals resulting from AEs.
Part D
All 24 participants in Part D received single doses of the novel device 0.45mg and the Nicorette® Inhalator (10mg). The mean (SD) weight of formulation inhaled from the novel device in Part D was 0.7074g (0.3028g), corresponding to 0.3961mg nicotine.
PK Analysis
Maximum Plasma Concentration
In Part B, the mean venous plasma nicotine concentration increased following administration of all three treatments. The mean venous plasma Cmax following administration of the novel device 0.45 and 0.67mg was 3.28 and 3.92ng/ml, respectively (Table 1). The mean Cmax following administration of the Nicorette® Inhalator (10mg) was 6.57ng/ml (Table 1).
Summary of Pharmacokinetic Parameters by Treatment
| . | Part B (N = 24) . | Part D (N = 24) . | |||
|---|---|---|---|---|---|
| Nicorette® Inhalator 10 mg . | Novel device 0.45mga . | Novel device 0.67mga . | Nicorette® Inhalator 10 mg . | Novel device 0.45 mg . | |
| Cmax (ng/ml) | 6.566 (2.965) | 3.284 (1.238) | 3.915 (1.640) | 7.628 (4.718) | 3.519 (1.378) |
| Tmax (min) | 38.0 (11.8) | 18.7 (8.6) | 19.2 (11.8) | 36.3 (12.4) | 21.0 (13.5) |
| AUClast (min × ng/ml) | 977.7 (498.7) | 430.8 (273.8) | 545.3 (334.4) | 991.5 (595.4) | 406.1 (298.9) |
| AUCall (min × ng/ml) | 987.7 (487.7) | 453.3 (259.0) | 563.0 (322.9) | 1002.6 (584.5) | 433.2 (284.6) |
| AUC0–10 (min × ng/ml) | 13.5 (9.9) | 18.4 (11.3) | 22.5 (13.2) | 14.2 (13.8) | 17.3 (13.0) |
| . | Part B (N = 24) . | Part D (N = 24) . | |||
|---|---|---|---|---|---|
| Nicorette® Inhalator 10 mg . | Novel device 0.45mga . | Novel device 0.67mga . | Nicorette® Inhalator 10 mg . | Novel device 0.45 mg . | |
| Cmax (ng/ml) | 6.566 (2.965) | 3.284 (1.238) | 3.915 (1.640) | 7.628 (4.718) | 3.519 (1.378) |
| Tmax (min) | 38.0 (11.8) | 18.7 (8.6) | 19.2 (11.8) | 36.3 (12.4) | 21.0 (13.5) |
| AUClast (min × ng/ml) | 977.7 (498.7) | 430.8 (273.8) | 545.3 (334.4) | 991.5 (595.4) | 406.1 (298.9) |
| AUCall (min × ng/ml) | 987.7 (487.7) | 453.3 (259.0) | 563.0 (322.9) | 1002.6 (584.5) | 433.2 (284.6) |
| AUC0–10 (min × ng/ml) | 13.5 (9.9) | 18.4 (11.3) | 22.5 (13.2) | 14.2 (13.8) | 17.3 (13.0) |
Data are mean (SD). All novel device pharmacokinetic parameters except AUC0–10 were significantly different from the reference product (Nicorette® Inhalator 10mg) (p < .05). AUCall = area under the plasma concentration vs. time curve from time 0 to the end of the sample collection period; AUClast = area under the plasma concentration time curve from time 0 to the time of the last quantifiable concentration; AUC0–10 = area under the plasma concentration time curve from time 0 to 10min; Cmax = maximum plasma concentration; Tmax = time to maximum concentration.
aN = 23.
Summary of Pharmacokinetic Parameters by Treatment
| . | Part B (N = 24) . | Part D (N = 24) . | |||
|---|---|---|---|---|---|
| Nicorette® Inhalator 10 mg . | Novel device 0.45mga . | Novel device 0.67mga . | Nicorette® Inhalator 10 mg . | Novel device 0.45 mg . | |
| Cmax (ng/ml) | 6.566 (2.965) | 3.284 (1.238) | 3.915 (1.640) | 7.628 (4.718) | 3.519 (1.378) |
| Tmax (min) | 38.0 (11.8) | 18.7 (8.6) | 19.2 (11.8) | 36.3 (12.4) | 21.0 (13.5) |
| AUClast (min × ng/ml) | 977.7 (498.7) | 430.8 (273.8) | 545.3 (334.4) | 991.5 (595.4) | 406.1 (298.9) |
| AUCall (min × ng/ml) | 987.7 (487.7) | 453.3 (259.0) | 563.0 (322.9) | 1002.6 (584.5) | 433.2 (284.6) |
| AUC0–10 (min × ng/ml) | 13.5 (9.9) | 18.4 (11.3) | 22.5 (13.2) | 14.2 (13.8) | 17.3 (13.0) |
| . | Part B (N = 24) . | Part D (N = 24) . | |||
|---|---|---|---|---|---|
| Nicorette® Inhalator 10 mg . | Novel device 0.45mga . | Novel device 0.67mga . | Nicorette® Inhalator 10 mg . | Novel device 0.45 mg . | |
| Cmax (ng/ml) | 6.566 (2.965) | 3.284 (1.238) | 3.915 (1.640) | 7.628 (4.718) | 3.519 (1.378) |
| Tmax (min) | 38.0 (11.8) | 18.7 (8.6) | 19.2 (11.8) | 36.3 (12.4) | 21.0 (13.5) |
| AUClast (min × ng/ml) | 977.7 (498.7) | 430.8 (273.8) | 545.3 (334.4) | 991.5 (595.4) | 406.1 (298.9) |
| AUCall (min × ng/ml) | 987.7 (487.7) | 453.3 (259.0) | 563.0 (322.9) | 1002.6 (584.5) | 433.2 (284.6) |
| AUC0–10 (min × ng/ml) | 13.5 (9.9) | 18.4 (11.3) | 22.5 (13.2) | 14.2 (13.8) | 17.3 (13.0) |
Data are mean (SD). All novel device pharmacokinetic parameters except AUC0–10 were significantly different from the reference product (Nicorette® Inhalator 10mg) (p < .05). AUCall = area under the plasma concentration vs. time curve from time 0 to the end of the sample collection period; AUClast = area under the plasma concentration time curve from time 0 to the time of the last quantifiable concentration; AUC0–10 = area under the plasma concentration time curve from time 0 to 10min; Cmax = maximum plasma concentration; Tmax = time to maximum concentration.
aN = 23.
Results for Part D were similar to those for Part B, with the Nicorette® Inhalator (10mg) producing a higher, but later, peak than the novel device. The mean Cmax following administration of the novel device 0.45mg and Nicorette® Inhalator (10mg) was 3.52 and 7.63ng/ml, respectively (Table 1).
Time to Cmax
The mean nicotine Cmax calculated in Part B was higher following administration of the Nicorette® Inhalator (10mg) than either dose from the novel device (Table 1). However, the Tmax for the Nicorette® Inhalator (10mg) was longer than for the novel device of either strength (38.0min vs. 18.7 and 19.2min [novel device 0.45 and 0.67mg, respectively] in Part B and 36.3min vs. 21.0min [novel device 0.45 mg] in Part D) (Table 1).
Mean plasma nicotine concentrations over time by treatment for Parts B and D are shown in Supplementary Figures S2 and Supplementary Data.
Area Under the Concentration–Time Curve
In Part B, the mean AUC was higher for the Nicorette® Inhalator (10mg) than for the novel device of either strength. Similar results were seen in Part D (Table 1).
Comparison of the relative bioavailability of nicotine between treatments in both Parts B and D indicated that the novel device produced significantly lower Cmax, AUClast, and AUCall and a significantly shorter Tmax than the Nicorette® Inhalator (10mg) (Table 1). Analysis of the AUC0–10 from both Parts B and D confirmed the early delivery of higher amounts of nicotine from the novel device compared with the Nicorette® Inhalator (10mg).
Efficacy
Craving VAS Scores
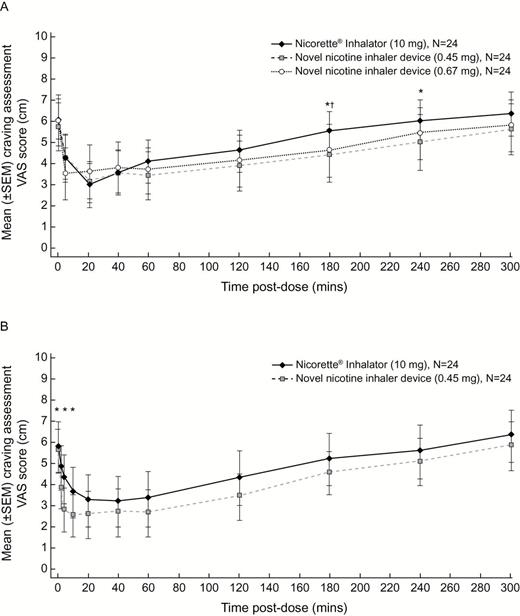
Mean craving scores assessed by the VAS were lower (higher craving relief) for the novel device than the Nicorette® Inhalator (10mg) at the majority of timepoints in both Part B (Figure 1A) and Part D (Figure 1B). In Part B, the lowest score was at 20min for both the novel device 0.45mg and the Nicorette® Inhalator (10mg) and at 4min for the novel device 0.67mg (Figure 1A). In Part D, the lowest mean craving VAS score was at 20min for the novel device 0.45mg and at 40min for the Nicorette® Inhalator (10mg) (Figure 1B).
Mean craving Visual Analog Scale (VAS) scores over time by treatment in (A) Part B and (B) Part D. *Difference between the novel nicotine inhaler device 0.45mg and Nicorette® Inhalator (10mg) is significant (p ≤ .05). †Difference between the novel nicotine inhaler device 0.67mg and Nicorette® Inhalator (10mg) is significant (p ≤ .05). SEM = standard error of the mean.
A comparison of the mean craving VAS scores showed that, in Part B, mean scores were lower (higher craving relief) for the novel device 0.45mg than for the Nicorette® Inhalator (10mg) at seven of the eight postdose timepoints and that this difference reached statistical significance at the 180- and 240-min timepoints (Figure 1A). Mean scores were lower for the novel device 0.67mg than for the Nicorette® Inhalator (10mg) at six of eight postdose timepoints, reaching statistical significance at the 180-min timepoint (Figure 1A). In Part D, mean scores were lower for the novel device 0.45mg than for the Nicorette® Inhalator (10mg) at all 10 postdose timepoints, differences reaching significance at the 2-, 4-, and 10-min timepoints (Figure 1B).
AUC for Craving VAS Scores
In both Parts B and D, the mean AUC was lower (indicating greater craving relief) for the novel device than the Nicorette® Inhalator (10mg). In Part B, the mean (SD) AUC for craving VAS score was lowest for the novel device 0.45mg (1356.3 [789.4] cm × min), followed by the novel device 0.67mg (1431.6 [769.0] cm × min). The greatest AUC was calculated for the Nicorette® Inhalator (10mg) (1566.3 [620.4] cm × min). In Part D, the mean AUC for craving VAS score was lower for the novel device 0.45mg (1208.5 [724.4] cm × min) than for the Nicorette® Inhalator (10mg) (1402.3 [815.2] cm × min).
A statistical comparison of craving AUC between treatments showed that in Part B, the AUC for the novel device 0.45mg was significantly lower than for the Nicorette® Inhalator (10mg) (p = .029); in Part D, the AUC for the novel device 0.45mg was lower than that for the Nicorette® Inhalator (10mg) and approached statistical significance (p = .059). These results suggest that greater relief of craving is achieved with both the medium and high doses of the novel device than with the Nicorette® Inhalator (10mg).
QSU-Brief Score
For both parts, the mean QSU-Brief total scores were reduced (indicating craving relief) at the time of first measurement (20min) for all treatment groups; similar patterns were seen for each of the QSU-Brief component scores for “anticipation” and “desire” (data not shown).
A treatment comparison of mean QSU-Brief scores showed that in Part B, total scores were statistically significantly lower for the novel device 0.45mg than for the Nicorette® Inhalator (10mg) at 120, 180, and 240min postdose (all p ≤ .05). In Part D, total scores were lower for the novel device although none of the differences were statistically significant (p > .05).
Safety
In Part B, a total of 87 TEAEs were reported by 23/24 (96%) participants. Of these, 79 were considered related to the study medication (Table 2). Most TEAEs were mild in nature. Two TEAEs were moderate, one of which was related to study medication (local numbness, which was reported 4min after administration of the inhalator and resolved within 15min). There was one report of mild numbness 20min postdose with the novel device 0.67mg. For both the novel device and Nicorette® Inhalator, mild tingling was the most commonly reported local tolerability symptom (Table 2).
Most Common TEAEs (Occurring in ≥5% of Participants in Any Treatment Group) by Treatment
| System organ class . | Part B . | Part D . | |||||
|---|---|---|---|---|---|---|---|
| Preferred term . | Novel device 0.45mg (N = 23) . | Novel device 0.67mg (N = 23) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . | Novel device 0.45mg (N = 24) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . |
| Subjects (%) with at least one TEAE | 17 (74) | 21 (91) | 14 (58) | 23 (96) | 15 (63) | 20 (83) | 22 (92) |
| Nervous system disorders | 3 (13) | 2 (9) | 6 (25) | 5 (21) | 3 (13) | 7 (29) | |
| Dizziness | 2 (9) | 3 (13) | 2 (8) | 2 (8) | |||
| Headache | 2 (9) | 3 (13) | 2 (8) | 3 (13) | |||
| Respiratory, thoracic, and mediastinal disorders | 7 (30) | 10 (43) | 8 (33) | 15 (63) | 3 (13) | 7 (29) | 8 (33) |
| Dry throat | 2 (8) | 2 (8) | |||||
| Pharyngeal erythema | 2 (9) | 2 (8) | |||||
| Throat irritation | 5 (22) | 6 (26) | 7 (29) | 11 (46) | 5 (21) | 5 (21) | |
| Gastrointestinal disorders | 12 (52) | 14 (61) | 9 (38) | 21 (88) | 12 (50) | 15 (63) | 19 (79) |
| Dry mouth | 2 (8) | ||||||
| Hypoesthesia oral | 3 (13) | 2 (8) | 6 (25) | 2 (8) | 3 (13) | ||
| Lip pain | 2 (8) | ||||||
| Nausea | 2 (8) | 2 (8) | |||||
| Paresthesia oral | 10 (43) | 9 (39) | 7 (29) | 15 (63) | 11 (46) | 14 (58) | 17 (71) |
| Skin and subcutaneous tissue disorders | 2 (8) | ||||||
| General disorders and administration site conditions | 3 (13) | 2 (9) | 2 (8) | 6 (25) | 2 (8) | ||
| Catheter site pain | 3 (13) | ||||||
| System organ class . | Part B . | Part D . | |||||
|---|---|---|---|---|---|---|---|
| Preferred term . | Novel device 0.45mg (N = 23) . | Novel device 0.67mg (N = 23) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . | Novel device 0.45mg (N = 24) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . |
| Subjects (%) with at least one TEAE | 17 (74) | 21 (91) | 14 (58) | 23 (96) | 15 (63) | 20 (83) | 22 (92) |
| Nervous system disorders | 3 (13) | 2 (9) | 6 (25) | 5 (21) | 3 (13) | 7 (29) | |
| Dizziness | 2 (9) | 3 (13) | 2 (8) | 2 (8) | |||
| Headache | 2 (9) | 3 (13) | 2 (8) | 3 (13) | |||
| Respiratory, thoracic, and mediastinal disorders | 7 (30) | 10 (43) | 8 (33) | 15 (63) | 3 (13) | 7 (29) | 8 (33) |
| Dry throat | 2 (8) | 2 (8) | |||||
| Pharyngeal erythema | 2 (9) | 2 (8) | |||||
| Throat irritation | 5 (22) | 6 (26) | 7 (29) | 11 (46) | 5 (21) | 5 (21) | |
| Gastrointestinal disorders | 12 (52) | 14 (61) | 9 (38) | 21 (88) | 12 (50) | 15 (63) | 19 (79) |
| Dry mouth | 2 (8) | ||||||
| Hypoesthesia oral | 3 (13) | 2 (8) | 6 (25) | 2 (8) | 3 (13) | ||
| Lip pain | 2 (8) | ||||||
| Nausea | 2 (8) | 2 (8) | |||||
| Paresthesia oral | 10 (43) | 9 (39) | 7 (29) | 15 (63) | 11 (46) | 14 (58) | 17 (71) |
| Skin and subcutaneous tissue disorders | 2 (8) | ||||||
| General disorders and administration site conditions | 3 (13) | 2 (9) | 2 (8) | 6 (25) | 2 (8) | ||
| Catheter site pain | 3 (13) | ||||||
TEAE = treatment-emergent adverse event.
Most Common TEAEs (Occurring in ≥5% of Participants in Any Treatment Group) by Treatment
| System organ class . | Part B . | Part D . | |||||
|---|---|---|---|---|---|---|---|
| Preferred term . | Novel device 0.45mg (N = 23) . | Novel device 0.67mg (N = 23) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . | Novel device 0.45mg (N = 24) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . |
| Subjects (%) with at least one TEAE | 17 (74) | 21 (91) | 14 (58) | 23 (96) | 15 (63) | 20 (83) | 22 (92) |
| Nervous system disorders | 3 (13) | 2 (9) | 6 (25) | 5 (21) | 3 (13) | 7 (29) | |
| Dizziness | 2 (9) | 3 (13) | 2 (8) | 2 (8) | |||
| Headache | 2 (9) | 3 (13) | 2 (8) | 3 (13) | |||
| Respiratory, thoracic, and mediastinal disorders | 7 (30) | 10 (43) | 8 (33) | 15 (63) | 3 (13) | 7 (29) | 8 (33) |
| Dry throat | 2 (8) | 2 (8) | |||||
| Pharyngeal erythema | 2 (9) | 2 (8) | |||||
| Throat irritation | 5 (22) | 6 (26) | 7 (29) | 11 (46) | 5 (21) | 5 (21) | |
| Gastrointestinal disorders | 12 (52) | 14 (61) | 9 (38) | 21 (88) | 12 (50) | 15 (63) | 19 (79) |
| Dry mouth | 2 (8) | ||||||
| Hypoesthesia oral | 3 (13) | 2 (8) | 6 (25) | 2 (8) | 3 (13) | ||
| Lip pain | 2 (8) | ||||||
| Nausea | 2 (8) | 2 (8) | |||||
| Paresthesia oral | 10 (43) | 9 (39) | 7 (29) | 15 (63) | 11 (46) | 14 (58) | 17 (71) |
| Skin and subcutaneous tissue disorders | 2 (8) | ||||||
| General disorders and administration site conditions | 3 (13) | 2 (9) | 2 (8) | 6 (25) | 2 (8) | ||
| Catheter site pain | 3 (13) | ||||||
| System organ class . | Part B . | Part D . | |||||
|---|---|---|---|---|---|---|---|
| Preferred term . | Novel device 0.45mg (N = 23) . | Novel device 0.67mg (N = 23) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . | Novel device 0.45mg (N = 24) . | Nicorette® Inhalator 10mg (N = 24) . | Total (N = 24) . |
| Subjects (%) with at least one TEAE | 17 (74) | 21 (91) | 14 (58) | 23 (96) | 15 (63) | 20 (83) | 22 (92) |
| Nervous system disorders | 3 (13) | 2 (9) | 6 (25) | 5 (21) | 3 (13) | 7 (29) | |
| Dizziness | 2 (9) | 3 (13) | 2 (8) | 2 (8) | |||
| Headache | 2 (9) | 3 (13) | 2 (8) | 3 (13) | |||
| Respiratory, thoracic, and mediastinal disorders | 7 (30) | 10 (43) | 8 (33) | 15 (63) | 3 (13) | 7 (29) | 8 (33) |
| Dry throat | 2 (8) | 2 (8) | |||||
| Pharyngeal erythema | 2 (9) | 2 (8) | |||||
| Throat irritation | 5 (22) | 6 (26) | 7 (29) | 11 (46) | 5 (21) | 5 (21) | |
| Gastrointestinal disorders | 12 (52) | 14 (61) | 9 (38) | 21 (88) | 12 (50) | 15 (63) | 19 (79) |
| Dry mouth | 2 (8) | ||||||
| Hypoesthesia oral | 3 (13) | 2 (8) | 6 (25) | 2 (8) | 3 (13) | ||
| Lip pain | 2 (8) | ||||||
| Nausea | 2 (8) | 2 (8) | |||||
| Paresthesia oral | 10 (43) | 9 (39) | 7 (29) | 15 (63) | 11 (46) | 14 (58) | 17 (71) |
| Skin and subcutaneous tissue disorders | 2 (8) | ||||||
| General disorders and administration site conditions | 3 (13) | 2 (9) | 2 (8) | 6 (25) | 2 (8) | ||
| Catheter site pain | 3 (13) | ||||||
TEAE = treatment-emergent adverse event.
In Part D, a total of 61 TEAEs were reported by 22/24 (92%) participants. Of these, 19 reported a total of 50 TEAEs that were considered related to the study medication and all were mild (Table 2). For both the novel device and inhalator, mild tingling was the most commonly reported local tolerability symptom (Table 2).
All AEs seen in Parts B and D were mild or moderate in nature and none were reported as severe. There were no serious AEs (SAEs) or deaths throughout the study, and no participants discontinued treatment because of an AE. Overall, the most common AEs were oral paresthesia, throat irritation, headache, and oral hypoesthesia. In terms of local tolerability, the most common local AE reported by participants in both parts of the study was tingling of the mouth/lips.
There were no clinically significant changes in mean vital signs over time for the duration of the study.
Discussion
We present the first set of data from this clinical study assessing a novel nicotine inhaler device. Although these evaluations represent only two parts of the four-part study, they report comparative analyses of the novel device versus the Nicorette® Inhalator (10mg). Specifically, both compared the 0.45mg nicotine dose of the novel device with the Nicorette® Inhalator (10mg) with (generally) consistent results for this dose level across the two parts, strengthening the conclusions drawn.
The PK data show a more rapid appearance of nicotine in venous blood after administration of nicotine from the novel device than after administration of the Nicorette® Inhalator (10mg). The overall nicotine exposure (AUC) from the Nicorette® Inhalator (10mg) was approximately twice that of the novel device, and the nicotine Cmax was higher with the Nicorette® Inhalator (10mg) than with either dose from the novel device, in line with the different doses delivered by each device.
The shorter venous nicotine Tmax for the novel device compared with the inhalator supports the hypothesis that a proportion of the dose from the novel device is delivered by a faster route (pulmonary deposition) than the oromucosal route via the Nicorette® Inhalator (10mg).5,16 The rapid appearance of nicotine in arterial blood after oral inhalation from the novel device (Moyses et al.15, poster presented at SRNT) relative to that in venous blood is in contrast to the profile found with the Nicorette® Inhalator, where there is a lower Cmax observed in arterial than venous blood.18
The lack of bioequivalence between the novel device and the reference product, Nicorette® Inhalator (10mg), was expected given their differences in delivered dose. The Nicorette® Inhalator (10mg) contains 10mg of nicotine (of which approximately 4mg is available for inhalation)5 in comparison with a dose delivered by the novel device that contains approximately 0.45 or 0.67mg of nicotine.
Despite a clear difference in nicotine exposure between the novel device and the Nicorette® Inhalator (10mg), analysis of the craving VAS AUC suggests that craving was more satisfied with the novel device than the Nicorette® Inhalator (10mg), the efficacy of which has been demonstrated in previous trials.19–21 In Part B, the 0.45mg dose of nicotine from the novel inhaler device had a significantly greater effect on craving AUC than the Nicorette® Inhalator (10mg) (p = .029), and in Part D, this approached statistical significance (p = .059), indicating a reproducible effect. Mean craving VAS scores were slightly higher for the Nicorette® Inhalator (10mg) than for either dose of the novel device; again, this suggests that reduction in craving may have been less with the Nicorette® Inhalator (10mg).
Efficacy was also measured using the QSU-Brief to assess smoking urges. The results supported the craving VAS scores, with QSU-Brief scores reduced using the novel device compared with the Nicorette® Inhalator (10mg). In Part B, between 2- and 4-hr postdose, the 0.45mg dose of the novel device had a statistically significant greater effect on QSU-Brief scores than the Nicorette® Inhalator (10mg). The results were similar in Part D although the difference did not reach statistical significance.
Administration of nicotine via the novel device was well tolerated by the healthy volunteers who participated in this study, with an AE profile similar to the Nicorette® Inhalator (10mg). The most common AEs were oral paresthesia, throat irritation, headache, and oral hypoesthesia, which showed no clear dose–effect relationship. Oropharyngeal sensations, including “throat scratch,” may be viewed as enhancing the respiratory cues associated with smoking by some individuals, rather than regarded as unpleasant.22–24
Comparison of venous plasma nicotine concentrations shows that the time course of nicotine delivery from a single cigarette is markedly different to that from currently available NRT products,5 which deliver nicotine more slowly. A standard cigarette delivers 1–2mg nicotine, which is rapidly absorbed into the pulmonary circulation. This leads to a rapid rise in arterial nicotine concentration and nicotine reaches the brain within seconds. Currently available forms of NRT may fail to help many smokers quit, as their nicotine PK profiles differ to smoking a cigarette.4 In this study, craving reduction was at least as great following nicotine administration with the novel device as the reduction following administration with the Nicorette® Inhalator (10mg), despite a lower overall nicotine exposure. The marked reduction in craving may well result from the close replication of behavioral aspects of smoking, combined with a rapid delivery of nicotine and respiratory tract sensory cues. Although the Nicorette® Inhalator (10mg) is also an inhaler device and mimics the hand-to-mouth action of smoking with upper airway sensory stimulation, nicotine is released in the vapor phase, leading to pronounced buccal deposition of nicotine. Nicotine absorption from the buccal mucosa has been shown to be slower than from pulmonary deposition.25 In this study, the venous peak in nicotine concentration occurred more rapidly with the novel device than with the Nicorette® Inhalator (10mg). Although the time to peak venous nicotine concentration with the novel device 0.45mg was longer than that reported for smoking (11.7 [Part B] and 21.0 [Part D] vs. 11.9min),26 the overall results of this study, nevertheless, suggest that the novel nicotine inhaler device could potentially be a more satisfying and acceptable alternative to cigarettes than the Nicorette® Inhalator (10mg). More appealing nicotine-containing products are regarded as being important for reducing the prevalence of smoking in the future, reducing the harm caused by tobacco-based nicotine addiction and addressing the associated health implications.8,9
This investigation has some limitations that should be acknowledged. A dose anomaly may have affected the PK results in Part B of the study. Following a review of laboratory testing of the novel device during the early phase of the study, it transpired that the quantity of nicotine delivered via the novel device for the first dose (i.e., for one complete refill) tended to be significantly lower (at 70%) than the nicotine delivered in subsequent doses. This anomaly may have affected data from Part B as PK testing was performed following the first refill of the novel device. This first-dose anomaly was avoided in Part D by testing the fourth refill. Results between the two parts were similar despite this discrepancy.
In Parts B and D of this study, PK analysis was carried out using venous blood samples and some of the conclusions drawn are based on an extrapolation of the results to the arterial PK profile.
Although this small, short-duration study was not designed to fully evaluate safety, AEs were collected systematically and the trial was conducted according to Good Clinical Practice. Both products were well tolerated with similar AE rates and profiles, all consistent with expectations for oral/inhaled NRT.
In conclusion, nicotine inhalation with the novel nicotine inhaler device provided greater craving relief than with the Nicorette® Inhalator (10mg), despite lower total nicotine exposure. Assessment of craving and smoking urges showed that craving reduction by the novel device was at least as great as the active comparator and statistically superior at the majority of timepoints, which was also shown in an overall craving AUC analysis. This is possibly owing to the novel device achieving a combination of nicotine PK and sensorial profiles that closely resemble those of a cigarette. This novel nicotine inhaler device has the potential to confer additional benefits in smoking cessation.
Funding
This study was supported by Kind Consumer Limited, London, UK. Nicoventures Ltd, London, UK, provided development funding in return for the license to the final product. Nicoventures is part of the British American Tobacco Group but is separate from the tobacco businesses.
Declaration of Interests
CM and AH are employees of Kind Consumer Limited. AR has no conflicts of interest to disclose.
Acknowledgments
The authors thank Linda Brown, BSc (Hons), of Caudex Medical (Oxford, UK), funded by Nicoventures Ltd, for assistance with the preparation of the initial draft of the manuscript, collating author comments, and assembling tables and figures. The authors also wish to acknowledge the following individuals for critical review during the development of this manuscript: Ritika Gupta, MSc, DIC, and Zeid Bsaibes, MA, of Kind Consumer Limited, and Kevin Bridgman, MB, ChB, MRCGP, FFPM, of Nicoventures Ltd, and also Linear Clinical Research who conducted the study, Clinical Network Services who acted as local sponsor, and CPR Pharma Services who provided bioanalytical and statistical support.
References




Comments