-
PDF
- Split View
-
Views
-
Cite
Cite
Marcel G M Olde Rikkert, René J F Melis, Alan A Cohen, G M E E (Geeske) Peeters, Why illness is more important than disease in old age, Age and Ageing, Volume 51, Issue 1, January 2022, afab267, https://doi.org/10.1093/ageing/afab267
Close - Share Icon Share
Abstract
Clinical reasoning and research in modern geriatrics often prioritises the disease concept. This is understandable as it has brought impressive advances in medicine (e.g. antibiotics, vaccines, successful cancer treatment and many effective surgeries). However, so far the disease framework has not succeeded in getting us to root causes of many age-related chronic diseases (e.g. Alzheimer’s disease, diabetes, osteoarthritis). Moreover, in aging and disease constructs alone fail to explain the variability in illness presentations.
Therefore, we propose to apply the underused illness concept in a new way by reconsidering the importance of common symptoms in the form of a dynamic network of symptoms as a complementary framework. We show that concepts and methods of complex system thinking now enable to fruitfully monitor and analyse the multiple interactions between symptoms in such in networks, offering new routes for prognosis and treatment. Moreover, close attention to the symptoms that bother older persons may also improve weighing the therapeutic objectives of well-being and survival and aligning treatment targets with the patients’ priorities.
Key Points
The disease framework has not succeeded in identifying root causes of many age-related chronic diseases.
The illness concept can be operationalised by developing dynamic symptom networks presenting the individual patient’s symptoms and their interactions.
Complex systems thinking and computational methods now enable application of dynamic symptom networks in clinical practice.
A new symptom network illness concept may assist geriatric patients and their physicians in shared goal setting and decision-making.
Introduction
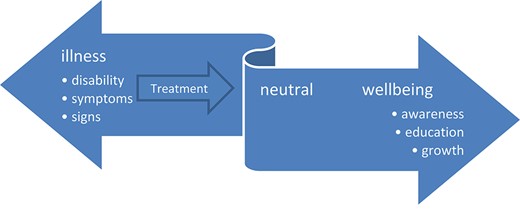
It is not only 50 years since Age and Ageing started its crescent road as a practical and scholarly journal, but also 50 years since public health physician John Travis developed his dynamic illness–well-being continuum (Figure 1) [1]. He stated that understanding the connections between disease, illness and well-being is a prerequisite for improving well-being. This is crucial in geriatric care for frail older patients who often prioritise improved functioning and well-being over survival [2]. However, as physicians, even as geriatricians, we are often focusing more on the illness side of the illness–well-being model than on the well-being side. And while focusing on the pathology, we have been taught to diagnose and treat the underlying diseases.
The illness–wellness continuum proposes that individuals can move dynamically to the right, towards greater health and well-being, passing through the stages of awareness, education and growth in self-management, coming from illness states with a variety of clinical signs, symptoms and disabilities. (adapted from ref 1.)
Around the birth of Age and Ageing, the late Marshall Marinker, intellectual giant in general practice, suggested a helpful way of distinguishing disease, illness and sickness. He characterised ‘three modes of unhealth’ [3]. Marinker recognised the objectivity of disease which doctors are able to see, hear, smell, feel, . . . and diagnose, and defined it as the pathological process, clearly deviant from the biological norm, most often physical as in a throat infection, or cancer of the bronchus, but sometimes undetermined in origin, as in schizophrenia. Currently, the disease model is dominant in medicine, but in aging research and geriatrics there are profound reasons to extend it (BOX 1). Therefore, it is very valuable to also remember the complementary concepts of illness and sickness. Marinker defined ‘illness’ as a feeling, an experience of unhealth which is entirely personal, interior to the person of the patient, often accompanying disease, but the disease may still be undeclared, as in the early stages of cancer or tuberculosis or diabetes. Marinker completed his triad with sickness as the social role, status and negotiated external and public mode of an unhealthy position in the outside world. The person is called ‘sick’, and society is prepared to recognise and sustain him.
Limitations of the disease constructs (DC) as dominant conceptual model in geriatric medicine
1) DC increasingly fail to explain the illness presentations in aging individuals and multimorbidity.
2) >DC make us focus on treatment/cure rather than prevention and generalised health.
3) Many diseases are etiologically unclear (e.g. schizophrenia, Alzheimer’s).
4) Many diseases are continuous processes rather than fully discrete (e.g. hypertension, diabetes, dementia) causing problems with the disease–health dichotomy.
5) Occam’s one-at-a-time approach to disease obviously ignores the complexity of multiple interacting (patho)physiological processes.
6) Causal chains are complex, leading to issues in defining disease. Is inflamm-aging a disease, or is it the cause of or contributing factor to separate diseases (diabetes, cancer, heart disease, etc.)?
7) Many important aspects of health (e.g. resilience) cannot be classified in a disease framework.
Since then, traditional medical education has put diagnosis of disease on the foreground pushing illness behaviour to the background. Diseases are valued as the central facts in medical practice, illness was reframed as the subjective experience of a disease and clinical management prioritised disease modification above symptom clarification and alleviation. This is in line with the central position of Occam’s razor in medicine urging to determine the single underlying cause that explains the symptoms a patient presents with [4]. What does not fit in this explanation is often ignored as irrelevant to the case at hand. However, geriatricians recognised that illness is primarily what motivates older persons to see a physician [3]. In line with this, illness behaviour was recognised as a dynamic process of adaptation to and coping with the diseases, both highly relevant for recovery [5].
About the same time in the seventies, Susan Sontag, experienced her breast cancer treatment and lobbied with the media and physicians to stop addressing patients’ illnesses in metaphors. In her shared decision-making talks she had to deal with professionals who did not use the ‘c word’, but spoke of a ‘battle to be won’ and ‘the challenging journey to go’. In response, Sontag wrote, ‘that illness is not a metaphor, and that the most truthful way of regarding illness—and the healthiest way of being ill—is one most purified of, most resistant to, metaphoric thinking’ [6].
Now, almost 50 years later, we feel that it is still relevant to accept Susan Sontag’s challenge to purify the illness concept. In this paper, we will draw on non-metaphorical theoretical and practical language to elucidate the illness concept. This is especially relevant concerning older persons, who often experience multiple threatening symptoms such as loneliness, pain, anxiety, fatigue and loss of function and autonomy. We will try to refocus on illness through the lens of complex system theory, which extends the perspective of evidence-based medicine (EBM). The latter at the start was meant to be a synthesis of available evidence, patient preferences and clinical experience, which altogether requires a very complex synthesis [7]. However, EBM is dominantly translated into clinical practice as a reductionist search for trial-based evidence. Complexity science theory and methods reach beyond such trial-based evidence that focuses on simplified dichotomized research questions, and can be complementary by disentangling interacting multiple symptoms, and delivering new treatment targets.
In this viewpoint, we will first address the limitations of common diagnostic reasoning in conformity with the traditional biomedical model to explain illness behaviour of older people when it is related to multimorbidity or geriatric symptoms. Next, we introduce complex systems concepts that may very well fit application in geriatric medicine. We show that these concepts can improve our understanding of key-characteristics of geriatric patients, for which we are indebted to the wealth of geriatric knowledge presented over time [8].
Single disease paradigm
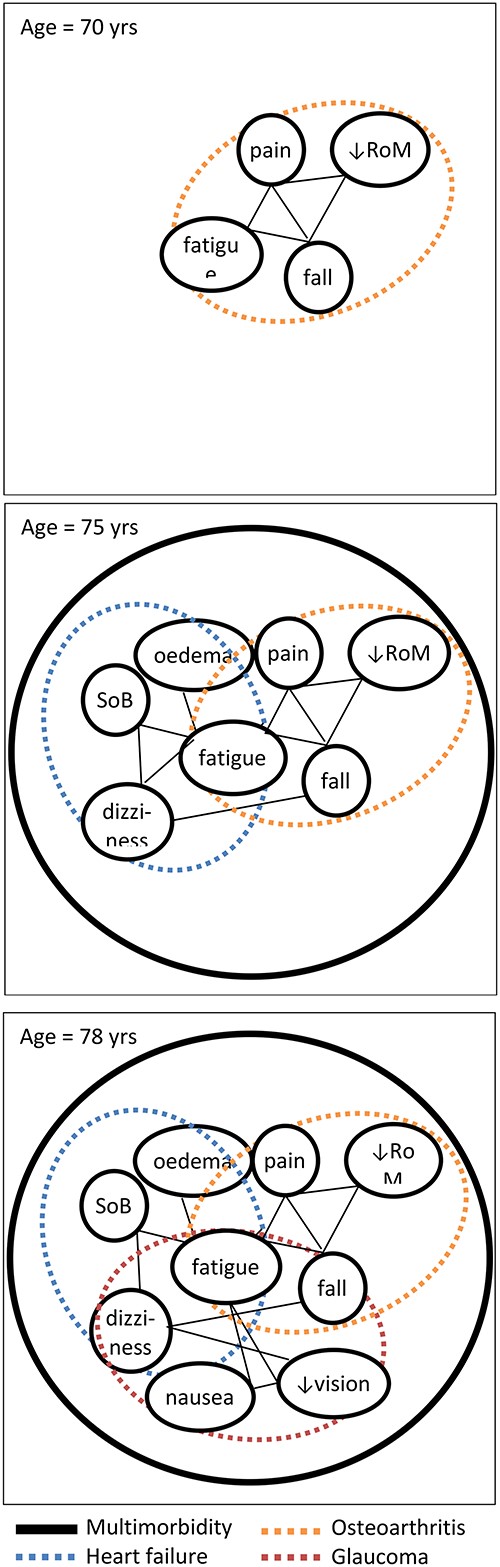
Currently the single disease paradigm is still dominant in medicine in general, and also plays a major role in geriatric reasoning. This paradigm (sometimes referred to as Occam’s razor) aims to explain illness by looking at patients´ symptoms (subjective: e.g. pain) and clinical signs (objective: e.g. high blood pressure) in specific patient episodes and by linking these with a single disease [9, 10]. Thus, clinicians aim to identify the single best cause for a patient’s constellation of symptoms (Figure 2). Diagnostic reasoning in geriatrics should however take into account the high prevalence of multimorbidity, which increases with age from around 10% at the age of 40 to 85% in those aged 75 and over [11]. In older adults with multimorbidity, a single symptom may arise from multiple diseases (Figure 2: e.g. fatigue may arise from both heart failure and osteoarthritis). Parallel treatment of single diseases easily leads to a high total treatment burden, over-treatment and aggravation of disease burden due to drug–drug, drug–disease and drug–nutrition interactions [12–14]. Thus, in case of multimorbidity, the cumulative single disease approach is often inefficient and potentially harmful [12, 15]. Probably, the single-disease thinking in old age should be restricted predominantly to patients with acute diseases, though these frequently may also be explained by an acute deterioration of already known chronic diseases. Moreover, the single disease paradigm often is a curative paradigm in which effectiveness of treatment is typically expressed in terms of cure and increased survival. However, ill older patients with multimorbidity often benefit more from a paradigm that focuses on symptom relief and functional recovery [2].
A hypothetical symptom network for a patient who over time develops osteoarthritis (panel/time 1 at 70 years), next gets heart failure (panel/time 2 at 75 years), and finally glaucoma (panel/time 3 at 78 years) Dotted ovals reflect chronic disease constructs; ‘nodes’ reflect symptoms and signs; straight lines (‘edges’) represent relationships between these symptoms and signs, which can be positive (aggravating symptoms); or negative (alleviating). RoM, range of motion; SoB, shortness of breath.
Diagnostic reasoning with multimorbidity
Despite the great urgency, geriatric medicine still lacks a valid and clinically applicable model for adequate diagnosis, prognosis and treatment of multimorbidity [15]. Commonly used epidemiological methods try to explain multimorbidity pathophysiology by using sum scores, morbidity indices, and clustering of diseases. Clinically, multimorbidity is taken into account by cumulative (sometimes weighted) comorbidity scores, if considered at all. However, these epidemiology-based methods all fail to capture the dynamics and complexity of multimorbidity and its impact on the individual patient. Moreover, these methods are quantitative, abstract figures that do not inform clinical decision-making and thus are of limited added value in clinical practice. Even the most advanced models still rely on clustering of single disease concepts [16], and do not explain the interactions in multiple organ systems [4].
In clinical practice comprehensive geriatric assessment in general includes a basic list of problems and diseases, often supplemented with other lists for differential diagnoses, but tools that explain the interrelatedness of diseases are lacking. Consequently, diagnostic reasoning and quality of care for people with multimorbidity is limited by a poor understanding of interactions between diseases, symptoms and impairments over time. Moreover, with increasing disease burden, presenting symptoms and signs become less specific and more difficult to pin down to a clear set of underlying diseases. This is why specificity of so many diagnostic tests (e.g. d-dimer, troponin, pro-BNP) is rather low in geriatric patients, making disease prediction for differential diagnoses more difficult. Geriatricians have recognised this as atypical disease presentation [6]. Despite poor fit with illness presentations in older persons, the diagnostic disease quest is often leading at all disease stages. This disease oriented approach may be explain acute diseases or diseases that are remediable, but is far less relevant in chronic diseases without proven disease modifying drugs [17].
Geriatric syndromes and illnesses
An alternative way to handle multimorbidity is to group symptoms according to their relation to geriatric syndromes. Geriatric syndromes are defined as mostly atypical acute (e.g. falls, acute confusion) or chronic symptoms (e.g. dizziness, incontinence) in older persons, predominantly explained by individual sets of multiple component causes [9]. In essence, geriatric syndromes lump symptoms, risk factors and diseases, but often do not consider their interactions at full relevance.
This geriatric syndrome reasoning probably is not most fruitful in the management of many chronic conditions and at later stages of patients’ illness trajectories, such as in end stage heart failure or chronic arthritis. Like the single disease paradigm, the clinical reasoning with geriatric syndromes currently still prioritises the quest for (new) diagnoses and ‘ruling out’ of the most feared diseases (e.g. cancer), even with low a priori diagnostic probability. This is mainstream geriatric syndrome teaching and practice. The limited or absent added value of looking for new diagnoses is explained by the competition between already known diseases, lack of disease modifying drugs, and the patients’ priority of well-being and alleviating symptoms. The critical question we should ask ourselves is: how important is it to precisely determine the nature of the underlying disease when one is helping an older person cope with illness? Answering this question may be helped by introducing complex systems thinking.
Complex systems thinking
Complex systems thinking implies that illness in case of multimorbidity is not caused by a simple sum of single diseases and may offer an alternative explanatory model to the biomedical model. The science serving this field is devoted to understanding the general properties of complex systems. Core hallmarks of complex systems include: [1] networks of interacting elements (e.g. interactions among aging mechanisms such as oxidative stress and amyloid aggregation in dementia or decreased mobility, depressed mood and joint pain), [2] feedback/feedforward loops (e.g. adaptive loops such as blood pressure regulation and maladaptive loops such as higher inflammatory states in Alzheimer’s disease or older COVID-19 patients), [3] a multiscale or modular hierarchical structure (e.g. accumulating cellular damage nested within organ tissue, within organisms and families), [4] non-linear dynamics (e.g. tipping points in disease trajectories that cause acute flipping from dementia to a delirious state) and [5] emergent properties: the sum of properties of system components is not equal or even similar to the whole system outcome (e.g. well-being and illness cannot be understood simply as the sum of multiple morbidities as we diagnose them when they occur individually). Here we focus on the implications these concepts can have when illness in frail older persons is regarded as a dynamic symptom network (DSN).
Illness as DSN
Complexity science methods can be of diagnostic value by offering the theory and modelling methods of interactive networks. This may allow clinicians and researchers to develop individualised dynamic network models in which interactions and overlap between symptoms and signs across diseases are mapped out and changes in symptom clustering over time are tracked (Figure 3). Such a model may also facilitate timely recognition, diagnosis and management of the outcome scenario differentially fitting the progressing patient journey: curing underlying disease(s), improving functional performance, alleviating symptoms or comforting dying.
Panel 1Dynamic symptom network, reflecting the symptoms evolution over time of Mrs. M (81 years), with health-related depressed feelings, osteoarthritis, heart failure, hypertension and fear of falling. Now, she asks for help because of bad sleep by joint pain, which causes increasing and interacting complaints. In diagnostic and therapeutic reasoning the dynamic symptom network orientation can be additive in improving her functioning and well-being (e.g. good pain relieve improves sleep, and sadness probably will best be served training mobility and cardiovascular condition instead of adding an antidepressant [19].) Panel 2 Interactive symptom network: a complexity-informed approach that can elucidate the interactions in clinical case management of Mrs M. Note: many different factors influence clinical case presentation, including multiple interactions of NSAIDs. They are contributing (+, green), counteracting (−, red) depending on the context [23].
We therefore propose to develop clinical DSN-based using principles of complexity science and network analysis. First evidence for the clinical utility of this approach comes from mental health research, in which the single disease model often fails and complex psychological symptom networks have advanced understanding and treatment of mental disorders [18]. We already showed that quantifying symptom interactions over time has prognostic meaning and may point at the most promising target symptoms in frail but also non-frail older adults [19, 20]. Figure 3 shows two panels that can visualise DSNs. Panel 1 highlights the changing interactions over time between these symptoms, and panel 2 may be read as a symptom interaction diagram that can be loaded with positive or negative feedback loops between the symptoms. Such interaction diagrams may be elaborated in computational models (e.g. quantified system dynamics models), which can be used for simulations of therapeutic actions. Such computational models already have been used successfully to predict epileptic seizures and hypoglycaemia [21, 22]. Development and implementation of these models requires monitoring changes of target symptoms over time. This technology, for example by using smart phones, is currently sufficiently advanced and simple to be also used by frail older patients. Moving forward, DSNs might also be developed to include different layers, such as fixed factors (genetic predispositions, medical antecedents) and potential interventions.
We note that DSNs are expected to also have some limitations: they do not, for example, explicitly incorporate upstream etiological factors, and are thus an incomplete representation of the true networks determining health. They also will not fully replace disease thinking, which has had major successes in many contexts. Rather, we see DSNs as a complementary tool that can inform a new way of thinking, particularly in the context of geriatrics or other complex health problems. DSNs may be a first step towards developing context-specific medical paradigms to supplement the single disease paradigm. Other approaches might include a global conception of health and a paradigm to better cover the ensemble of symptoms, illnesses and disease.
Conclusion
The dominance of multimorbidity in geriatric patients necessitates further evolution of clinical reasoning and scientific evaluation methods. We still want to understand and improve illness behaviour, but only reasoning in disease constructs is too reductionist. Complexity science offers promising tools that can extend this clinical reasoning. DSNs may form the foundation of a new paradigm to understand and treat geriatric illness episodes and trajectories. These should not replace geriatric syndromes or disease thinking, but may have a strong synergistic value, as thinking in symptoms and an illness concept may be more closely related to improving well-being outcomes in older patients.
In future research, DSNs may be used to: (i) understand the complex, time-varying interrelations of symptoms, signs and diseases; (ii) develop prognostic models for changes in symptoms, signs and diseases over time; and (iii) evaluate effects of therapeutic interventions on the total symptom burden. However, first much research is needed to develop and evaluate clinical application of DSNs and to test whether they may guide geriatric medicine to more effective and less burdensome personalised health care.
Declaration of Conflicts of Interest
AAC is founder and CEO at Oken Health.
Declaration of Sources of funding
None.



![Panel 1Dynamic symptom network, reflecting the symptoms evolution over time of Mrs. M (81 years), with health-related depressed feelings, osteoarthritis, heart failure, hypertension and fear of falling. Now, she asks for help because of bad sleep by joint pain, which causes increasing and interacting complaints. In diagnostic and therapeutic reasoning the dynamic symptom network orientation can be additive in improving her functioning and well-being (e.g. good pain relieve improves sleep, and sadness probably will best be served training mobility and cardiovascular condition instead of adding an antidepressant [19].) Panel 2 Interactive symptom network: a complexity-informed approach that can elucidate the interactions in clinical case management of Mrs M. Note: many different factors influence clinical case presentation, including multiple interactions of NSAIDs. They are contributing (+, green), counteracting (−, red) depending on the context [23].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ageing/51/1/10.1093_ageing_afab267/5/m_afab267f3.jpeg?Expires=1716381249&Signature=0z4xCVMajVFE1O4f8tunu8JycB96y5BnkzeRQAv7tqGOpvRUe0yAZ39BvBCQODKe-T~Jb1WbkKS0EsrB1s2dXdMKzF9H37Q12oClegXVE46xBEMhJFF1PUzuNua2Zr1f6PsdxsJod4P7UxuydXfLXYxCzNxdog0-ilcYtqWjvXJ5oMjpsDoErFGmifw1jcEqQ1a1Umd8jwUOH1lEhZvkqnHXmxsqpqziyM3P9fnHNgOEQ29U44XaFJxW~fYtW1lUutTmloGuHXBTWBH1tdJMaPT50tA0Koucyp5CAJkJQvhEAYKeB4f-HtGNN5hr4nv6WAFxH9vV5XOGoeQOJSmJJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments