-
PDF
- Split View
-
Views
-
Cite
Cite
Felipe Martinez, Catalina Tobar, Nathan Hill, Preventing delirium: should non-pharmacological, multicomponent interventions be used? A systematic review and meta-analysis of the literature, Age and Ageing, Volume 44, Issue 2, March 2015, Pages 196–204, https://doi.org/10.1093/ageing/afu173
Close - Share Icon Share
Abstract
Background: delirium is a complex neuropsychiatric syndrome that is common among elderly inpatients. It has been associated with increased mortality, longer hospital stays, cognitive and functional decline and increased institutionalisation rates. Multicomponent interventions, a series of non-pharmacological strategies frequently handled by nursing staff, might be useful for prevention.
Objectives: to assess the efficacy of multicomponent interventions in preventing incident delirium in the elderly.
Methods: a systematic review of randomised trials was undertaken. Two independent reviewers performed iterative literature searches in seven databases without language restrictions. Grey literature repositories were considered as well. The quality of included trials was assessed by using the criteria established by the Cochrane Collaboration. When possible, data were synthesised into a meta-analysis. Heterogeneity was assessed using the χ2 and I2 tests.
Findings: a total of 21,788 citations were screened, and seven studies of diverse quality were included in the review, comprising 1,691 participants. Multicomponent interventions significantly reduced incident delirium (relative risk [RR] 0.73, 95% confidence interval [CI] 0.63–0.85, P < 0.001) and accidental falls during the hospitalisation (RR 0.39, 95% CI 0.21, 0.72, P = 0.003), without evidence of differential effectiveness according to ward type or dementia rates. Non-significant reductions in delirium duration, hospital stay and mortality were found as well.
Interpretation: multicomponent interventions are effective in preventing incident delirium among elderly inpatients. Effects seemed to be stable among different settings. Due to the limited amount of data, potential benefits in survival need to be confirmed in further studies. Future research should be aimed at contrasting different multicomponent programmes to select the most useful interventions.
Introduction
Delirium is a complex neuropsychiatric syndrome characterised by acute and fluctuating onset, inattention, altered level of consciousness and evidence of disorganised thinking [1]. It is common in patients on acute wards, with prevalence often ranging between 10 and 31% [2]. This diversity may be explained by different risk factor prevalences co-existing with the syndrome, such as age, cognitive impairment, alcohol abuse or multiple co-morbid conditions [3–5]. Severity of disease is also a factor that partially explains why delirium is frequent in acute wards, Intensive Care Units (ICUs) [6], and among patients undergoing extensive inpatient procedures (e.g. hip fracture repair or cardiac surgery) [1].
Delirium is associated with adverse outcomes, such as increased mortality [6, 7], accidental falls [8, 9], cognitive decline [10], functional dependence [11] and increased healthcare costs [12]. Multicomponent interventions (MIs) have been proposed as prevention strategies to cope with delirium as the risk factors that coalesce to initiate delirium syndrome are heterogeneous. MI components comprise measures aimed at reducing predisposing factors for developing delirium: physical therapy, cognitive stimulation programmes, nutritional supplementation, among others are usual components within MIs [13, 14]. Several practice guidelines [15, 16] have encouraged the establishment of MI programmes to prevent incident delirium. For instance, NICE guidelines [17] recommend that patients be assessed for risk of delirium within 24 h of admission and tailored MI be implemented. Specific elements include cognitive stimulation, avoidance of dehydration, promotion of good sleep patterns, nutritional support and early mobilisation. Methodological limitations have hampered the reliability of this evidence. Therefore, we seek to reappraise the available information regarding the clinical effectiveness of MIs in preventing incident delirium.
Methods
Systematic review
The protocol has been published (PROSPERO:CRD42013003736) and conducted in accordance with PRISMA guidelines [18]. Two independent reviewers performed iterative searches, without language restrictions, in PubMed/MEDLINE, EMBASE, PsycInfo, CINAHL, Cochrane Library, Cochrane Register for Controlled Trials (CENTRAL), LILACS, SciELO and grey literature repositories (OpenGrey, Clinicaltrials.gov) using the Cochrane Collaboration's Highly Sensitive Search Strategy to optimise search results [19]. Relevant search terms included Delirium, Post-Operative Delirium, Acute Confusion, Acute Confusional Syndrome, Metabolic Encephalopathy, Brain Diseases, Metabolic, Brain Failure, Acute Brain Failure, Exogenous Psychosis, Clouded State, Clouding of Consciousness, Toxic Psychosis, Toxic Confusion, Multicomponent Interventions, Multidisciplinary Care Teams, Multidisciplinary Team Interventions, Non-Pharmacologic Interventions, Clocks, Calendars, Safe Environment, Occupational Therapy, Activities of Daily Living, Family Counselling, Family Involvement, Education, Nutrition Assessment, Nutrition Therapy, Drug Review, Drug Utilisation Reviews, Fluid Therapy, Hearing Aids and Lenses. Databases were searched from inception to 31 December 2012. Reference lists of guidelines [15–17, 20, 21] were hand-searched for potentially relevant trials. Disagreements were solved by discussion, and an arbiter (NRH) was used where consensus could not be reached.
Randomised trials contrasting MIs to usual care in preventing incident delirium were eligible for inclusion. Interventions having components in at least two of the following domains were considered to be an MI [22]: physical interventions including hydration (fluid therapy), electrolyte and nutrition, safe environment directives, drug reviews, cognitive stimulation programmes, daily reorientation activities, educational interventions for staff and family members, family involvement in patient care and physical or occupational therapy during hospital stay. Usual care was defined as standard care given to patients within their wards, including use of medication to treat symptoms arising from delirium, and interventions specific to correction of underlying causes when already present. Elderly patients are at increased vulnerability to develop delirium so studies conducted in populations >60 years were selected. Diagnosis of delirium was on standardised criteria, as recommended by NICE [17]. A minimum follow-up of 24 h was considered as a requirement to account for sundowning (increasing symptoms often seen among patients with the condition). Observational and non-randomised studies were excluded, as were trials evaluating management of prevalent delirium, those aimed at alcohol withdrawal delirium (delirium tremens) and trials assessing pharmacological interventions.
Trial quality was evaluated using the criteria proposed by the Cochrane Collaboration [19], which included appropriateness of randomisation, concealment of allocation sequences, level of blinding used, losses to follow-up and a check regarding whether analyses were performed under the intention-to-treat principle. An additional category ‘Other sources of bias’ was included for reviewers to highlight methodological concerns that went beyond the aforementioned criteria. Quality assessments were performed independently by two reviewers (C.T. and F.M.).
Data collection
Data were extracted independently using standardised forms (by C.T. and F.M.). Data included quality assessment, method used to diagnose delirium, clinical setting, types of interventions, patient characteristics and outcomes. Authors of articles were contacted to provide additional information whenever necessary.
Statistical analysis
When appropriate, data were summarised into meta-analysis. The primary outcome for this systematic review was development of incident delirium at any point during hospitalisation. Delirium duration, length of hospitalisation, accidental falls, institutionalisation rates and in-hospital 3-, 6- and 12-month mortality were considered as secondary end points. Heterogeneity was expected as this review addresses different combinations of interventions and diverse clinical scenarios in which delirium develops [23]. Random effects model was selected to summarise trial results. Heterogeneity was assessed using Cochrane's Q and the I2 statistics. The I2 heterogeneity was categorised as follows: <25% low, 25–50% moderate and >50% high. With low-level heterogeneity, a fixed effects model was used to pool results. Sources of heterogeneity were assessed with pre-specified subgroup analyses according to dementia rates and clinical setting. Publication bias was evaluated through funnel plot and Egger's test. Analyses were undertaken in Review Manager (RevMan-v5.2). Authors had access to all data and take responsibility for the integrity and the accuracy of results.
Results
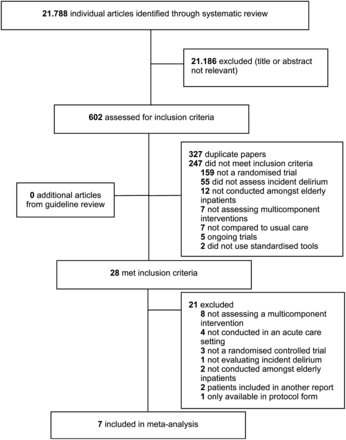
The initial search yielded 21,788 articles and 602 were considered for the review. After abstract assessment, 28 articles met the inclusion criteria and were selected for full-text review. No additional citations were found by examining guideline's references. Seven studies met selection criteria comprising 1,691 participants. Among these, one manuscript was published by two of this review's authors and was sent to the arbiter to ensure appropriateness. Breakdown of study selection process is given in Figure 1.
The seven manuscripts detailed three trials in orthopaedic ward patients with hip fractures [14, 24, 25], two trials in acute medical wards [26, 27], one trial in coronary care [28] and one trial in intensive care [29]. Trials assessed different intervention strategies, but specific components were shared. Physiotherapy was most common, in 70% of trials [14, 24, 25, 27, 29]. Other interventions included daily reorientation (60%) [14, 26, 28], family involvement in care (60%) [25, 26, 28, 29], stimulation programmes with avoidance of sensorial deprivation (60%) and staff/family member education–(40%) [24, 26, 28]. Only one trial specifically addressed drug review [14], and one mentioned its use among participants in both study arms [29]. The Confusion Assessment Method (CAM) was the most frequently used diagnostic technique (90%) [14, 25–29]. Two trials excluded patients with dementia at baseline [28, 29], with wide ranges of prevalence in the remaining trials (6–40%) [14, 24–27]. In six trials [14, 24, 25, 27–29], intervention was carried out by a trained team of physiotherapists, nutritionists, social care workers, nurses, occupational therapists and physicians. In the remaining trial [26], family members carried out the intervention after a brief training session. Individual trial characteristics are given in Table 1.
Individual study characteristics
| Study . | n . | Method of assessment . | Setting . | Patient characteristics . | Main intervention characteristics . | Overall risk of bias . |
|---|---|---|---|---|---|---|
| Alvarez et al. [29] | 64 | CAM | Intensive care unit | Elderly inpatients admitted to an ICU. Patients with dementia, language impairments, need for mechanical ventilation or limited life expectancies (<90 days) were excluded. | For up to 5 days, patients received cognitive and sensorial stimulation, training in activities of daily living, positioning, upper body stimulation and family involvement in care. Interventions were provided in 40-min sessions twice daily. | Moderate |
| Finotto et al. [28] | 48 | CAM | Coronary care unit | Those with dementia, Parkinson's disease or psychiatric co-morbidities were excluded. | The multicomponent intervention comprised safe environment directives, reorientation, avoidance of sensorial deprivation and extended family visits. Additionally, family members received education on delirium. | High |
| Jeffs et al. [27] | 648 | CAM | Medical ward | Patients with severe dysphasia, life expectancy of <24 h, isolation for infection control, contraindication to mobilisation, planned admission <48 h, major psychiatric diagnosis, prevalent delirium and those transferred from other hospitals were excluded. Eighty-eight patients (13.6%) had dementia at baseline | The intervention comprised an exercise and orientation programme. Sessions were held twice daily in 20- to 30-min sessions. Exercises were modified according to individual participant's abilities. Daily reorientation was performed daily in the form of questions among included patients. | Moderate |
| Lundström et al. [24] | 199 | OBS-scale MMSE | Orthopaedic ward | Patients with severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, pathological fractures or those bedridden prior to surgery were excluded. A diagnosis of dementia at baseline was found among 28 (27.5%) patients in the intervention group and 36 (37.1%) of patients belonging in the control arm. | The intervention strategy included staff education, prevention of complications, control of pain, sleep hygiene, nutrition programmes, early rehabilitation and mobilisation and reduction of invasive procedures (i.e. urinary catheterisation) | Moderate |
| Marcantonio et al. [14] | 126 | CAM | Orthopaedic ward | Eligible patients were admitted for hip surgery repair. Patients were excluded if they had a life expectancy to <6 months or an inability to provide informed consent within 24 h of surgery or 48 h of admission. Forty per cent of participants had a diagnosis of dementia at baseline. | Patients allocated to the intervention arm received a multicomponent programme comprising 10 modules. Interventions were tailored to patient needs in two to five specific recommendations. Main components included adequate CNS oxygen delivery, fluid and electrolyte balance, treatment of severe pain, elimination of unnecessary medications, regulation of bowel and bladder function, adequate nutritional intake, early mobilisation and rehabilitation, prevention, early detection and treatment of major post-operative complications, appropriate environmental stimuli and the treatment of agitated delirium. | Low |
| Martinez et al.[26] | 287 | CAM | Medical ward | ‘High-risk’ patients were randomised, as established by a clinical prediction rule. Patients with prevalent delirium were excluded, as were those patients without family support, those who were admitted to a ward different from general medicine, had been admitted to a room with more than two beds or refused participation. Six per cent had a diagnosis of dementia at baseline. | The intervention consisted of six components: family education on delirium, provision of clocks and calendars in the room, avoidance of sensorial deprivation, safe environment directives, reorientation of the patient as provided by family members and extended visitation times. | Moderate |
| Vidán et al. [25] | 319 | CAM | Orthopaedic ward | Candidate patients were admitted for hip surgery repair. Those with an inability to walk prior to the fracture, dependency in all basic activities of daily living, pathological hip fracture or terminal illnesses (life expectancy of <12 months) were excluded. Seventy-eight participants had a diagnosis of dementia (24.5%) at baseline. | A geriatric team that included a geriatrician, a rehabilitation specialist and a social worker met to define and correct included patient's problems. Physical therapy was included in the intervention arm. Specific components were not reported. | High |
| Study . | n . | Method of assessment . | Setting . | Patient characteristics . | Main intervention characteristics . | Overall risk of bias . |
|---|---|---|---|---|---|---|
| Alvarez et al. [29] | 64 | CAM | Intensive care unit | Elderly inpatients admitted to an ICU. Patients with dementia, language impairments, need for mechanical ventilation or limited life expectancies (<90 days) were excluded. | For up to 5 days, patients received cognitive and sensorial stimulation, training in activities of daily living, positioning, upper body stimulation and family involvement in care. Interventions were provided in 40-min sessions twice daily. | Moderate |
| Finotto et al. [28] | 48 | CAM | Coronary care unit | Those with dementia, Parkinson's disease or psychiatric co-morbidities were excluded. | The multicomponent intervention comprised safe environment directives, reorientation, avoidance of sensorial deprivation and extended family visits. Additionally, family members received education on delirium. | High |
| Jeffs et al. [27] | 648 | CAM | Medical ward | Patients with severe dysphasia, life expectancy of <24 h, isolation for infection control, contraindication to mobilisation, planned admission <48 h, major psychiatric diagnosis, prevalent delirium and those transferred from other hospitals were excluded. Eighty-eight patients (13.6%) had dementia at baseline | The intervention comprised an exercise and orientation programme. Sessions were held twice daily in 20- to 30-min sessions. Exercises were modified according to individual participant's abilities. Daily reorientation was performed daily in the form of questions among included patients. | Moderate |
| Lundström et al. [24] | 199 | OBS-scale MMSE | Orthopaedic ward | Patients with severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, pathological fractures or those bedridden prior to surgery were excluded. A diagnosis of dementia at baseline was found among 28 (27.5%) patients in the intervention group and 36 (37.1%) of patients belonging in the control arm. | The intervention strategy included staff education, prevention of complications, control of pain, sleep hygiene, nutrition programmes, early rehabilitation and mobilisation and reduction of invasive procedures (i.e. urinary catheterisation) | Moderate |
| Marcantonio et al. [14] | 126 | CAM | Orthopaedic ward | Eligible patients were admitted for hip surgery repair. Patients were excluded if they had a life expectancy to <6 months or an inability to provide informed consent within 24 h of surgery or 48 h of admission. Forty per cent of participants had a diagnosis of dementia at baseline. | Patients allocated to the intervention arm received a multicomponent programme comprising 10 modules. Interventions were tailored to patient needs in two to five specific recommendations. Main components included adequate CNS oxygen delivery, fluid and electrolyte balance, treatment of severe pain, elimination of unnecessary medications, regulation of bowel and bladder function, adequate nutritional intake, early mobilisation and rehabilitation, prevention, early detection and treatment of major post-operative complications, appropriate environmental stimuli and the treatment of agitated delirium. | Low |
| Martinez et al.[26] | 287 | CAM | Medical ward | ‘High-risk’ patients were randomised, as established by a clinical prediction rule. Patients with prevalent delirium were excluded, as were those patients without family support, those who were admitted to a ward different from general medicine, had been admitted to a room with more than two beds or refused participation. Six per cent had a diagnosis of dementia at baseline. | The intervention consisted of six components: family education on delirium, provision of clocks and calendars in the room, avoidance of sensorial deprivation, safe environment directives, reorientation of the patient as provided by family members and extended visitation times. | Moderate |
| Vidán et al. [25] | 319 | CAM | Orthopaedic ward | Candidate patients were admitted for hip surgery repair. Those with an inability to walk prior to the fracture, dependency in all basic activities of daily living, pathological hip fracture or terminal illnesses (life expectancy of <12 months) were excluded. Seventy-eight participants had a diagnosis of dementia (24.5%) at baseline. | A geriatric team that included a geriatrician, a rehabilitation specialist and a social worker met to define and correct included patient's problems. Physical therapy was included in the intervention arm. Specific components were not reported. | High |
Individual study characteristics
| Study . | n . | Method of assessment . | Setting . | Patient characteristics . | Main intervention characteristics . | Overall risk of bias . |
|---|---|---|---|---|---|---|
| Alvarez et al. [29] | 64 | CAM | Intensive care unit | Elderly inpatients admitted to an ICU. Patients with dementia, language impairments, need for mechanical ventilation or limited life expectancies (<90 days) were excluded. | For up to 5 days, patients received cognitive and sensorial stimulation, training in activities of daily living, positioning, upper body stimulation and family involvement in care. Interventions were provided in 40-min sessions twice daily. | Moderate |
| Finotto et al. [28] | 48 | CAM | Coronary care unit | Those with dementia, Parkinson's disease or psychiatric co-morbidities were excluded. | The multicomponent intervention comprised safe environment directives, reorientation, avoidance of sensorial deprivation and extended family visits. Additionally, family members received education on delirium. | High |
| Jeffs et al. [27] | 648 | CAM | Medical ward | Patients with severe dysphasia, life expectancy of <24 h, isolation for infection control, contraindication to mobilisation, planned admission <48 h, major psychiatric diagnosis, prevalent delirium and those transferred from other hospitals were excluded. Eighty-eight patients (13.6%) had dementia at baseline | The intervention comprised an exercise and orientation programme. Sessions were held twice daily in 20- to 30-min sessions. Exercises were modified according to individual participant's abilities. Daily reorientation was performed daily in the form of questions among included patients. | Moderate |
| Lundström et al. [24] | 199 | OBS-scale MMSE | Orthopaedic ward | Patients with severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, pathological fractures or those bedridden prior to surgery were excluded. A diagnosis of dementia at baseline was found among 28 (27.5%) patients in the intervention group and 36 (37.1%) of patients belonging in the control arm. | The intervention strategy included staff education, prevention of complications, control of pain, sleep hygiene, nutrition programmes, early rehabilitation and mobilisation and reduction of invasive procedures (i.e. urinary catheterisation) | Moderate |
| Marcantonio et al. [14] | 126 | CAM | Orthopaedic ward | Eligible patients were admitted for hip surgery repair. Patients were excluded if they had a life expectancy to <6 months or an inability to provide informed consent within 24 h of surgery or 48 h of admission. Forty per cent of participants had a diagnosis of dementia at baseline. | Patients allocated to the intervention arm received a multicomponent programme comprising 10 modules. Interventions were tailored to patient needs in two to five specific recommendations. Main components included adequate CNS oxygen delivery, fluid and electrolyte balance, treatment of severe pain, elimination of unnecessary medications, regulation of bowel and bladder function, adequate nutritional intake, early mobilisation and rehabilitation, prevention, early detection and treatment of major post-operative complications, appropriate environmental stimuli and the treatment of agitated delirium. | Low |
| Martinez et al.[26] | 287 | CAM | Medical ward | ‘High-risk’ patients were randomised, as established by a clinical prediction rule. Patients with prevalent delirium were excluded, as were those patients without family support, those who were admitted to a ward different from general medicine, had been admitted to a room with more than two beds or refused participation. Six per cent had a diagnosis of dementia at baseline. | The intervention consisted of six components: family education on delirium, provision of clocks and calendars in the room, avoidance of sensorial deprivation, safe environment directives, reorientation of the patient as provided by family members and extended visitation times. | Moderate |
| Vidán et al. [25] | 319 | CAM | Orthopaedic ward | Candidate patients were admitted for hip surgery repair. Those with an inability to walk prior to the fracture, dependency in all basic activities of daily living, pathological hip fracture or terminal illnesses (life expectancy of <12 months) were excluded. Seventy-eight participants had a diagnosis of dementia (24.5%) at baseline. | A geriatric team that included a geriatrician, a rehabilitation specialist and a social worker met to define and correct included patient's problems. Physical therapy was included in the intervention arm. Specific components were not reported. | High |
| Study . | n . | Method of assessment . | Setting . | Patient characteristics . | Main intervention characteristics . | Overall risk of bias . |
|---|---|---|---|---|---|---|
| Alvarez et al. [29] | 64 | CAM | Intensive care unit | Elderly inpatients admitted to an ICU. Patients with dementia, language impairments, need for mechanical ventilation or limited life expectancies (<90 days) were excluded. | For up to 5 days, patients received cognitive and sensorial stimulation, training in activities of daily living, positioning, upper body stimulation and family involvement in care. Interventions were provided in 40-min sessions twice daily. | Moderate |
| Finotto et al. [28] | 48 | CAM | Coronary care unit | Those with dementia, Parkinson's disease or psychiatric co-morbidities were excluded. | The multicomponent intervention comprised safe environment directives, reorientation, avoidance of sensorial deprivation and extended family visits. Additionally, family members received education on delirium. | High |
| Jeffs et al. [27] | 648 | CAM | Medical ward | Patients with severe dysphasia, life expectancy of <24 h, isolation for infection control, contraindication to mobilisation, planned admission <48 h, major psychiatric diagnosis, prevalent delirium and those transferred from other hospitals were excluded. Eighty-eight patients (13.6%) had dementia at baseline | The intervention comprised an exercise and orientation programme. Sessions were held twice daily in 20- to 30-min sessions. Exercises were modified according to individual participant's abilities. Daily reorientation was performed daily in the form of questions among included patients. | Moderate |
| Lundström et al. [24] | 199 | OBS-scale MMSE | Orthopaedic ward | Patients with severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, pathological fractures or those bedridden prior to surgery were excluded. A diagnosis of dementia at baseline was found among 28 (27.5%) patients in the intervention group and 36 (37.1%) of patients belonging in the control arm. | The intervention strategy included staff education, prevention of complications, control of pain, sleep hygiene, nutrition programmes, early rehabilitation and mobilisation and reduction of invasive procedures (i.e. urinary catheterisation) | Moderate |
| Marcantonio et al. [14] | 126 | CAM | Orthopaedic ward | Eligible patients were admitted for hip surgery repair. Patients were excluded if they had a life expectancy to <6 months or an inability to provide informed consent within 24 h of surgery or 48 h of admission. Forty per cent of participants had a diagnosis of dementia at baseline. | Patients allocated to the intervention arm received a multicomponent programme comprising 10 modules. Interventions were tailored to patient needs in two to five specific recommendations. Main components included adequate CNS oxygen delivery, fluid and electrolyte balance, treatment of severe pain, elimination of unnecessary medications, regulation of bowel and bladder function, adequate nutritional intake, early mobilisation and rehabilitation, prevention, early detection and treatment of major post-operative complications, appropriate environmental stimuli and the treatment of agitated delirium. | Low |
| Martinez et al.[26] | 287 | CAM | Medical ward | ‘High-risk’ patients were randomised, as established by a clinical prediction rule. Patients with prevalent delirium were excluded, as were those patients without family support, those who were admitted to a ward different from general medicine, had been admitted to a room with more than two beds or refused participation. Six per cent had a diagnosis of dementia at baseline. | The intervention consisted of six components: family education on delirium, provision of clocks and calendars in the room, avoidance of sensorial deprivation, safe environment directives, reorientation of the patient as provided by family members and extended visitation times. | Moderate |
| Vidán et al. [25] | 319 | CAM | Orthopaedic ward | Candidate patients were admitted for hip surgery repair. Those with an inability to walk prior to the fracture, dependency in all basic activities of daily living, pathological hip fracture or terminal illnesses (life expectancy of <12 months) were excluded. Seventy-eight participants had a diagnosis of dementia (24.5%) at baseline. | A geriatric team that included a geriatrician, a rehabilitation specialist and a social worker met to define and correct included patient's problems. Physical therapy was included in the intervention arm. Specific components were not reported. | High |
Included trials were considered to incur in a low-to-moderate risk of bias. Five trials gave evidence of adequate methods of randomisation and reported allocation methods as concealed from investigators [14, 24, 26, 27, 29]. The two remaining trials [25, 28] did not provide data regarding allocation concealment. All but a single trial [28] reported information about patient characteristics at baseline with no significant differences determined among study groups. Blinding was performed in all trials, and simple blinding of the outcome assessor was the most commonly used method. In four trials, blinding of participants was deemed impossible due to the characteristics and setting of intervention [14, 26, 28, 29], and blinding of the outcome assessors was performed in all but one trial [26]. In four trials [14, 24, 26, 27], an outcome analysis based on the intention-to-treat principle was reported, one trial did not provide information on analysis [28] and two trials [25, 29] used a per-protocol approach for analysis.
Evaluations for incident delirium were undertaken by trained clinical personnel in six trials, with no information regarding training from 1 trial [25]. Periodicity of examinations was not consistent across trials: daily assessments [14, 26], thrice daily evaluations [28], twice daily assessments up to 5 days after enrolment [29], systematic screenings every 48 h on weekdays [27] and single assessments 3–5 days after surgery followed by a prospective review for symptoms of delirium in clinical records that was performed three times per day [24]. In one trial, they did not provide data on the periodicity of evaluations regarding this outcome [25].
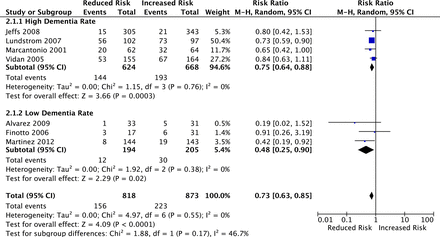
The analysis of incident delirium found that pooled meta-analysis for the seven trials (n = 1,619 patients) resulted in a relative risk of 0.73 (95% CI 0.63–0.85), with little evidence of heterogeneity (Cochrane's Q P = 0.55, I2 = 0%, Figure 2). No evidence of differential effectiveness was noted in pre-specified subgroup analyses.
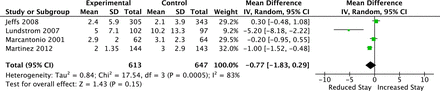
Delirium duration was analysed using data from four trials (n = 1,260). A non-significant reduction in delirium duration of 0.77 days (WMD −1.03, 95% CI −2.30, 0.23, P = 0.11, Figure 3) with evidence of significant heterogeneity (Cochrane's Q P < 0.001, I2 = 89%) was detected.
Six trials provided data regarding hospital length of stay. There were statistically significant reductions in duration of stay among patients allocated to MIs in two studies [24, 29], with no evidence in the other four. Pooled meta-analysis (n = 1,643) determined a small, non-significant reduction in length of stay (WMD −1.22 days, 95% CI −2.63, 0.20, P = 0.09, Supplementary data, Figure S5 available in Age and Ageing online). Significant heterogeneity was determined (P = 0.01, I2 = 66%) which was unlikely to be explained by the clinical setting in which trials were conducted (Test for subgroup differences P = 0.92).
In three trials [24, 25, 29], data on in-hospital mortality were provided (n = 582). These trials were conducted among elderly with highly noxious stimuli (critical illness and two participants with hip fractures). Among these, only Vidán et al.'s [25] study found a statistically significant benefit in preventing in-hospital deaths for patients allocated to the intervention. When trials were pooled, a non-significant reduction in in-hospital mortality was observed (RR 0.41, 95% CI 0.11–1.53, P = 0.19, I2 = 34% Cochrane's Q P = 0.22; Supplementary data, Figure S6 available in Age and Ageing online).
Limited data were available regarding institutionalisation with three studies considering nursing home placement among its outcomes. A descriptive approach was undertaken due to significant heterogeneity in trials. In Marcantonio et al.'s [14] trail, 92% of patients allocated to the intervention arm were discharged to an ‘institutional setting’, with a lower (88%) proportion in the control group. No significant differences were determined. Patients discharged to a nursing home were combined with those requiring continued care in a rehabilitation hospital, making this particular outcome impossible to assess with the data provided. Similar results were seen in Jeffs et al.' [27] study, where a non-significant increased number of patients returning to previous residence were reported among participants allocated to the intervention compared with controls (79% versus 75%, P = 0.40). Vidán et al.'s [25] trial considered site of discharge among its outcomes, yet no results were provided regarding this end point.
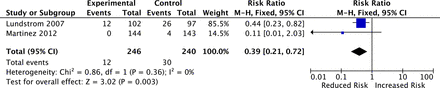
Data on the incidence of accidental falls during the hospital stay were available in two trials [30, 31] (n = 486). Pooled analysis determined incidence of falls as significantly reduced by intervention, with an RR of 0.39 (95% CI 0.21, 0.72, P = 0.003). No statistical evidence of heterogeneity (χ2, P = 0.36, I2 = 0%) was noted. Individual trial and pooled estimates are given in Figure 4.
The funnel plot (Supplementary data, Figure S7 available in Age and Ageing online) displayed slight asymmetry in favour of beneficial effect for reduction of incident delirium. Egger's test did not show evidence for publication bias (P = 0.14). Similar findings were seen for in-hospital mortality (P = 0.34), delirium duration (P = 0.35) and duration of stay (P = 0.28). Only two trials were available for pooling data on accidental falls; additional calculations could not be performed.
Discussion
This systematic review provides the first meta-analysis quantifying the effectiveness of MIs in preventing incident delirium among the elderly. A relative reduction of 30% in delirium rates was determined when using MIs regardless of setting and cognitive decline. This is in concordance with previous syntheses on the subject [32–34]. In spite of the inherent complexity in strategies, little evidence of differential effectiveness was seen among the included trials, even with subgroup analyses. Homogeneity might be explained in the commonality of individual interventions across programmes. Physical or occupational therapy, daily reorientation and the avoidance of sensorial deprivation were the most used interventions, suggesting a central role for these elements within complex multicomponent strategies. Due to the limited number of data, it was not possible to assess this by separate analyses without a significant risk of bias. MIs may provide a systematic approach to the correction of hospital-related triggering factors, such as inappropriate medications, immobility, sleep deprivation and loss of circadian rhythms [35, 36]. The correction of these factors implies a shared common pathway that may explain the observed constancy in treatment effects. Regardless of these considerations in this meta-analysis, MIs represent an effective method of preventing incident delirium, and therefore, their implementation should be considered among the standard of care for elderly inpatients.
Two trials assessed in-hospital falls; a significant reduction in this end point was found in the meta-analysis, with a pooled RR of 0.39 (95% CI 0.21, 0.72). It may be explained in the control of hyperactive symptoms, but due to the limited availability of data on delirium subtypes, a sub-analysis was not possible. Between 30 and 50% of hospital falls result in serious harms for patients and increase costs due to longer patient stays [37, 38]. Therefore, having an intervention with a potential to reduce falls may result in an improvement in hospital quality [39].
Non-significant reductions in delirium duration and length of stay were found, suggesting little role for these interventions when the condition is already present. These findings contradict previous findings with multicomponent strategies [40, 41] and a previous systematic review evaluating the role of acute geriatric units [42] that studied multicomponent programmes. This may be explained in part by the varied periodicity of evaluations seen among trials. Delirium is a highly fluctuating condition, and less frequent assessments might result in a biased estimation of the intervention's efficacy affecting the results. Conversely, it could be considered that early discharge planning is a common element within the model of care of acute geriatric units, and the aforementioned experiences were not necessarily seen within included protocols [43–45] Therefore, it is possible to speculate that the inclusion of this strategy within an MI could result in greater effects in reducing hospital stays. Further research is needed.
Trials with survival as an end point found a non-significant reduction in in-hospital mortality and no statistically significant effects when trials were pooled (RR 0.41, 95% CI 0.11, 1.53, P = 0.19). It should be considered that several well-designed, prospective cohort studies [6, 7] have consistently found a direct association of delirium with mortality. Hazard ratios have ranged between 2 and 3 even after adjustments for confounders. It may be that insufficient statistical power could explain the observed lack of significance. Future studies addressing MIs should consider adding survival as a major end point to allow definitive conclusions to be made.
The majority (85%) of trials provided basic information regarding individual strategies, yet information on how to implement them was limited. Some common elements were found that could facilitate implementation. They were informed multidisciplinary teamwork including hospital staff from different specialties and volunteers to undertake interventions. In all cases, basic training on how to deliver the intervention was described for participants. Training ranged from brief educational sessions on delirium for family members [26] to intensive courses for hospital staff [24]. In trials where hospital staff delivered the intervention, team roles were defined according to job-related skills, and trials established a leader to coordinate interventions. Several trials described flexibility within their protocols to allow tailoring of the intervention to the specific needs of individuals [14, 24, 25, 27, 28]. The commonality of implementation of these characteristics may suggest that they were devised to overcome expected difficulties. These insights are highlighted in previous experiences with MIs in delirium prevention [30, 40, 41, 46].
This meta-analysis is the most comprehensive and detailed update regarding multicomponent interventions in preventing incident delirium among the elderly. Its search strategy allowed the detection of several additional trials that had not been considered in previous systematic reviews [32–34]. Unpublished data provided additional information that was largely negative, and it may be the estimates that represent a conservative approximation of the intervention's effectiveness. The broad definition for MIs allowed the exploration of some heterogeneity, and the redundancy in several key steps of the review process made omissions of relevant information unlikely. This inclusive search strategy might result in an inappropriate comparison by allowing simpler MIs (two or three components) to be contrasted with more complex interventions. This concern is further elucidated when results from Jeffs et al.' [27] study are reviewed. Despite its considerable sample size, they found no evidence of beneficial effects for its simplified MI, in which two components (physical and cognitive therapy) were provided. However, it should be recognised that the median number of components among the included studies was six, and no evidence of statistical heterogeneity was found in the meta-analyses making the possibility of inappropriate comparisons unlikely. It is possible that the lack of significance that was observed in the Jeffs et al.' [27] study is explained by the monitoring strategy that was chosen, with patients being assessed every 48 h for delirium. Therefore, in spite of these limitations, this systematic review is the current best knowledge source in the area of MIs for prevention of delirium.
Sub-analyses for individual interventions within multicomponent programmes were not feasible given the limited number of trials. Limited information was available regarding specific implementation strategies and adherence rates, a phenomenon that seems to be frequent among randomised trials of non-pharmacological interventions [31]. This may influence results as adherence is likely to represent a crucial factor for success of an individual programme [30]. Furthermore, there were restricted data on key outcomes, such as mortality or site of discharge, that did not allow definitive conclusions. Publication biases cannot be excluded from conclusions due to the small number of trials, although it seems unlikely that harmful effects could arise from the implementation being tested.
Conclusions
This systematic review and meta-analysis found that MIs are effective in reducing incident delirium and reducing accidental falls in hospital and, thus, should be implemented as part of the standard of care for elderly inpatients. Some studies were at a moderate risk of bias, but the overall effect was consistent among individual trials. The effect of MIs did not differ according to clinical setting or dementia prevalence. Reductions in in-hospital mortality and length of hospital stay were seen in individual trials, but no statistically significant difference was determined. Future research should be undertaken aiming to contrast different MI programmes to select the most effective interventions. Further studies addressing the role of multicomponent interventions in functional outcomes, mortality and costs need to be undertaken.
Delirium is a common complication among elderly inpatients.
Multicomponent interventions are non-pharmacological strategies aimed at correcting risk factors for developing delirium.
Multicomponent interventions are effective in preventing incident delirium.
Conflicts of interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (i) F.M., C.T. and N.H. did not receive support for the submitted work; (ii) F.M., C.T. and N.H. have no relationships with companies that might have an interest in the submitted work in the previous 3 years; (iii) their spouses, partners or children have no financial relationships that may be relevant to the submitted work and (iv) F.M., C.T. and N.H. do not have non-financial interests that may be relevant to the submitted work. This study did not receive any funding.




Comments