-
PDF
- Split View
-
Views
-
Cite
Cite
Mitsuhiro Miyashita, Makoto Arai, Akiko Kobori, Tomoe Ichikawa, Kazuya Toriumi, Kazuhiro Niizato, Kenichi Oshima, Yuji Okazaki, Takeo Yoshikawa, Naoji Amano, Toshio Miyata, Masanari Itokawa, Clinical Features of Schizophrenia With Enhanced Carbonyl Stress, Schizophrenia Bulletin, Volume 40, Issue 5, September 2014, Pages 1040–1046, https://doi.org/10.1093/schbul/sbt129
Close - Share Icon Share
Abstract
Accumulating evidence suggests that advanced glycation end products, generated as a consequence of facilitated carbonyl stress, are implicated in the development of a variety of diseases. These diseases include neurodegenerative illnesses, such as Alzheimer disease. Pyridoxamine is one of the 3 forms of vitamin B6, and it acts by combating carbonyl stress and inhibiting the formation of AGEs. Depletion of pyridoxamine due to enhanced carbonyl stress eventually leads to a decrease in the other forms of vitamin B6, namely pyridoxal and pyridoxine. We previously reported that higher levels of plasma pentosidine, a well-known biomarker for advanced glycation end products, and decreased serum pyridoxal levels were found in a subpopulation of schizophrenic patients. However, there is as yet no clinical characterization of this subset of schizophrenia. In this study, we found that these patients shared many clinical features with treatment-resistant schizophrenia. These include a higher proportion of inpatients, low educational status, longer durations of hospitalization, and higher doses of antipsychotic medication, compared with patients without carbonyl stress. Interestingly, psychopathological symptoms showed a tendency towards negative association with serum vitamin B6 levels. Our results support the idea that treatment regimes reducing carbonyl stress, such as supplementation of pyridoxamine, could provide novel therapeutic benefits for this subgroup of patients.
Introduction
Schizophrenia is a debilitating disorder characterized with positive symptoms, such as auditory hallucinations and persecutive delusions, and negative symptoms, including emotional withdrawal and blunted affects. The lifetime prevalence is estimated at approximately 1%, and the onset of disease frequently occurs in early adulthood, making appropriate biological treatment and adequate psychosocial support essential to achieve and maintain a recovery. Although many studies have attempted to clarify the underlying disease mechanisms, the main cause and pathophysiology of schizophrenia remains unclear.
Carbonyl stress is an abnormal metabolic state, resulting from either an increased production of reactive carbonyl compounds (RCOs) through the oxidation of carbohydrates or by decreased detoxification of RCOs.1,2 Advanced glycation end products are generated as a consequence of facilitated carbonyl stress, and numerous experimental studies in animals and humans implicate increased advanced glycation end products in a variety of illnesses, including diabetes mellitus (DM), chronic kidney disease, cardiovascular diseases, and Alzheimer’s disease.2–5 Pyridoxamine, a form of vitamin B6, is capable of scavenging some RCOs, thereby inhibiting the formation of advanced glycation end products and alleviating ensuing unfavorable physiological effects. The other forms of vitamin B6, pyridoxine and pyridoxal, lack this therapeutic benefit, and exhaustion of pyridoxamine eventually leads to a decrease in these compounds.
We previously reported enhanced carbonyl stress as a feature in a subpopulation of schizophrenics.6 We noted a 1.7-fold higher mean plasma concentration of pentosidine, a well-known biomarker for advanced glycation end products, and significantly decreased mean serum pyridoxal levels in 45 schizophrenics, compared with 61 control subjects.6 However, to date, no studies have evaluated the clinical features of schizophrenics showing enhanced carbonyl stress. In this study, we investigated the clinical characteristics of this cohort and also assessed the association between the biomarkers, pentosidine and pyridoxal with psychopathological symptoms, evaluated using the Positive and Negative Syndrome Scale (PANSS). Characterizing the disease using candidate biomarkers is an effective approach for defining a relatively homogenous subpopulation from the heterogeneous schizophrenic population. In addition, clarifying clinical features specific to this subgroup of schizophrenics advances research into developing tailor-made medications suitable for these patients.
Material and Methods
Subjects
Patients, including 157 with schizophrenia and 6 with schizoaffective disorder, were diagnosed according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Diagnoses were made by at least 2 experienced psychiatrists. Patients with DM (hemoglobin A1c [HbA1c] ≥ 5.9), renal dysfunction (creatinine > 1.04 mg/dl for men and > 0.79 mg/dl for women or estimated glomerular filtration rate [eGFR] < 60.0 ml/min), Behcet’s disease, and chronic viral hepatitis type C were excluded from this study as these diseases are known to increase plasma pentosidine levels. Patients with a history of carcinoma within 1 year prior to blood sampling were also excluded for the same reason. Only 3 patients presented with acute exacerbation of symptoms at the time of blood sampling, whereas all other patients were in a chronic, stable state (see table 1). The schizophrenic subjects were divided into 4 groups according to their levels of the 2 enhanced carbonyl stress biomarkers; pentosidine and pyridoxal. The cutoff point for high plasma pentosidine levels was set at 62.9ng/ml, namely, the mean + 2 SDs of healthy controls, as determined in a previous report.6 Values under this threshold were defined as normal. Because serum pyridoxamine and pyridoxine were predictably under detection levels, we used serum pyridoxal levels as representative of serum vitamin B6. Decreased levels of serum vitamin B6 were defined as under 6ng/ml in male subjects and under 4ng/ml in female subjects. Likewise, low and normal pyridoxal levels were defined according to the cutoff point. The 4 groups were composed of a normal group (group 1), consisting of normal pentosidine and normal pyridoxal levels (28 males and 39 females), a low-pyridoxal group (group 2) with normal pentosidine and low pyridoxal (26 males and 7 females), a high-pentosidine group (group 3) with high pentosidine and normal pyridoxal (16 males and 21 females), and finally a group with enhanced carbonyl stress (group 4), showing high pentosidine and low pyridoxal levels (17 males and 9 females).
Clinical Features of Schizophrenics With and Without Carbonyl Stress
| . | Normal Pentosidine . | High Pentosidine . | ||
|---|---|---|---|---|
| Normal Pyridoxal . | Low Pyridoxal . | Normal Pyridoxal . | Low Pyridoxal . | |
| Group 1 . | Group 2 (Fold Change) . | Group 3 (Fold Change) . | Group 4 (Fold Change) . | |
| Number of subjects | 67 | 33 | 37 | 26 |
| Demographics | ||||
| Schizoaffective/schizophrenia | 3/64 | 0/33 (N/A) | 1/36 (0.60) | 2/24 (1.72) |
| Pentosidine (ng/ml) | 41.5±11.6 | 39.4±10.8 (0.95) | 127.3±98.3 (3.07)*** | 123.0±85.6 (2.97)*** |
| Pyridoxal (ng/ml) | 9.5±5.7 | 3.4±1.1 (0.36)*** | 7.9±3.2 (0.83) | 3.4±1.0 (0.36)*** |
| Age | 46.9±14.7 | 48.2±12.6 (1.03) | 49.4±10.5 (1.05) | 51.4±11.7 (1.10) |
| Sex (male/female) | 28/39 | 26/7 (1.89)** | 16/21 (1.03) | 17/9 (1.56) |
| HbA1C | 5.0±0.3 | 5.0±0.3 (1.00) | 5.0±0.2 (1.00) | 5.0±0.4 (1.00)a |
| eGFR (ml/min) | 83.9±13.8 | 87.6±14.4 (1.04) | 81.4±12.2 (0.97) | 86.3±15.4 (1.03)a |
| Cigarette smoking (+/−)b | 20/46 | 18/15 (1.80)* | 9/28 (0.80) | 11/15 (1.40) |
| Alcohol consumption (+/−)c | 17/45 | 6/26 (0.68) | 4/29 (0.44) | 3/23 (0.42) |
| GLO1 genotype | ||||
| Wild type/mutant type | 59/8 | 25/8 (0.86) | 29/8 (0.89) | 24/2 (1.05) |
| Glu111Ala | 7 | 8 | 8 | 0 |
| Ala111Ala | 1 | 0 | 0 | 1 |
| Frameshift (T27NfsX15) | 0 | 0 | 0 | 1 |
| GLO1 enzymatic activity (mU/106 RBC) | 7.06±0.77 | 7.03±0.99 (1.00) | 6.84±0.88 (0.97)a | 6.32±0.97 (0.90)a,** |
| Clinical variables | ||||
| Acute exacerbation/chronic state | 0/67 | 2/31 (N/A) | 1/36 (N/A) | 0/26 (N/A) |
| Inpatients/outpatients | 16/51 | 13/20 (1.65) | 23/14 (2.60)** | 21/5 (3.38)*** |
| Family history (+/−) | 26/41 | 9/24 (0.70) | 16/21 (1.11) | 8/18 (0.79) |
| Education duration (y) | 13.0±2.6 | 13.3±2.3 (1.02) | 12.3±3.0 (0.94) | 11.7±2.6 (0.90)* |
| Onset (y) | 25.6±8.6 | 24.7±7.4 (0.96) | 24.1±8.0 (0.94) | 25.0±10.8 (0.98)a |
| Disease duration (y) | 21.3±14.1 | 23.5±15.0 (1.10) | 25.3±11.7 (1.19) | 26.4±15.5 (1.24)a |
| Number of hospitalizations | 3.0±3.0 | 3.1±2.8 (1.04) | 4.2±3.5 (1.39) | 4.5±4.8 (1.49) |
| Hospitalization duration (y) | 4.2±9.2 | 7.7±12.9 (1.85) | 8.7±10.2 (2.08)* | 17.4±16.9 (4.17)** |
| Antipsychotics (mg/day; CP equivalent) | 773.8±652.4 | 931.8±677.2 (1.20) | 1162.9±810.7 (1.50)* | 1143.9±743.6 (1.48)* |
| . | Normal Pentosidine . | High Pentosidine . | ||
|---|---|---|---|---|
| Normal Pyridoxal . | Low Pyridoxal . | Normal Pyridoxal . | Low Pyridoxal . | |
| Group 1 . | Group 2 (Fold Change) . | Group 3 (Fold Change) . | Group 4 (Fold Change) . | |
| Number of subjects | 67 | 33 | 37 | 26 |
| Demographics | ||||
| Schizoaffective/schizophrenia | 3/64 | 0/33 (N/A) | 1/36 (0.60) | 2/24 (1.72) |
| Pentosidine (ng/ml) | 41.5±11.6 | 39.4±10.8 (0.95) | 127.3±98.3 (3.07)*** | 123.0±85.6 (2.97)*** |
| Pyridoxal (ng/ml) | 9.5±5.7 | 3.4±1.1 (0.36)*** | 7.9±3.2 (0.83) | 3.4±1.0 (0.36)*** |
| Age | 46.9±14.7 | 48.2±12.6 (1.03) | 49.4±10.5 (1.05) | 51.4±11.7 (1.10) |
| Sex (male/female) | 28/39 | 26/7 (1.89)** | 16/21 (1.03) | 17/9 (1.56) |
| HbA1C | 5.0±0.3 | 5.0±0.3 (1.00) | 5.0±0.2 (1.00) | 5.0±0.4 (1.00)a |
| eGFR (ml/min) | 83.9±13.8 | 87.6±14.4 (1.04) | 81.4±12.2 (0.97) | 86.3±15.4 (1.03)a |
| Cigarette smoking (+/−)b | 20/46 | 18/15 (1.80)* | 9/28 (0.80) | 11/15 (1.40) |
| Alcohol consumption (+/−)c | 17/45 | 6/26 (0.68) | 4/29 (0.44) | 3/23 (0.42) |
| GLO1 genotype | ||||
| Wild type/mutant type | 59/8 | 25/8 (0.86) | 29/8 (0.89) | 24/2 (1.05) |
| Glu111Ala | 7 | 8 | 8 | 0 |
| Ala111Ala | 1 | 0 | 0 | 1 |
| Frameshift (T27NfsX15) | 0 | 0 | 0 | 1 |
| GLO1 enzymatic activity (mU/106 RBC) | 7.06±0.77 | 7.03±0.99 (1.00) | 6.84±0.88 (0.97)a | 6.32±0.97 (0.90)a,** |
| Clinical variables | ||||
| Acute exacerbation/chronic state | 0/67 | 2/31 (N/A) | 1/36 (N/A) | 0/26 (N/A) |
| Inpatients/outpatients | 16/51 | 13/20 (1.65) | 23/14 (2.60)** | 21/5 (3.38)*** |
| Family history (+/−) | 26/41 | 9/24 (0.70) | 16/21 (1.11) | 8/18 (0.79) |
| Education duration (y) | 13.0±2.6 | 13.3±2.3 (1.02) | 12.3±3.0 (0.94) | 11.7±2.6 (0.90)* |
| Onset (y) | 25.6±8.6 | 24.7±7.4 (0.96) | 24.1±8.0 (0.94) | 25.0±10.8 (0.98)a |
| Disease duration (y) | 21.3±14.1 | 23.5±15.0 (1.10) | 25.3±11.7 (1.19) | 26.4±15.5 (1.24)a |
| Number of hospitalizations | 3.0±3.0 | 3.1±2.8 (1.04) | 4.2±3.5 (1.39) | 4.5±4.8 (1.49) |
| Hospitalization duration (y) | 4.2±9.2 | 7.7±12.9 (1.85) | 8.7±10.2 (2.08)* | 17.4±16.9 (4.17)** |
| Antipsychotics (mg/day; CP equivalent) | 773.8±652.4 | 931.8±677.2 (1.20) | 1162.9±810.7 (1.50)* | 1143.9±743.6 (1.48)* |
Note: HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate; GLO1, glyoxalase 1; CP, chlorpromazine; N/A, not applicable. Cutoff point for high plasma pentosidine levels = 62.9ng/ml (the mean + 2 SDs of healthy controls). Low pyridoxal levels: <6ng/ml (male) and <4ng/ml (female). Fold change (FC) = relative rate (RR) defined in group 1 as 1.00. For categorical variables, FC was calculated as follows: RR in groups 2–4/RR in group 1.
We conducted unpaired t test in comparison of groups 2–4 with group 1, ANCOVA (vs group 1, covariate = age), and Fisher’s Exact test for categorical variables.
aANCOVA (vs group1, covariate = age).
bLack of data for 1 patient in group 1.
cLack of data for 5, 1, and 4 patients in groups 1, 2, and 3, respectively.
*P < .05 (vs group 1), **P < .001(vs group 1), ***P < .0001(vs group 1).
Clinical Features of Schizophrenics With and Without Carbonyl Stress
| . | Normal Pentosidine . | High Pentosidine . | ||
|---|---|---|---|---|
| Normal Pyridoxal . | Low Pyridoxal . | Normal Pyridoxal . | Low Pyridoxal . | |
| Group 1 . | Group 2 (Fold Change) . | Group 3 (Fold Change) . | Group 4 (Fold Change) . | |
| Number of subjects | 67 | 33 | 37 | 26 |
| Demographics | ||||
| Schizoaffective/schizophrenia | 3/64 | 0/33 (N/A) | 1/36 (0.60) | 2/24 (1.72) |
| Pentosidine (ng/ml) | 41.5±11.6 | 39.4±10.8 (0.95) | 127.3±98.3 (3.07)*** | 123.0±85.6 (2.97)*** |
| Pyridoxal (ng/ml) | 9.5±5.7 | 3.4±1.1 (0.36)*** | 7.9±3.2 (0.83) | 3.4±1.0 (0.36)*** |
| Age | 46.9±14.7 | 48.2±12.6 (1.03) | 49.4±10.5 (1.05) | 51.4±11.7 (1.10) |
| Sex (male/female) | 28/39 | 26/7 (1.89)** | 16/21 (1.03) | 17/9 (1.56) |
| HbA1C | 5.0±0.3 | 5.0±0.3 (1.00) | 5.0±0.2 (1.00) | 5.0±0.4 (1.00)a |
| eGFR (ml/min) | 83.9±13.8 | 87.6±14.4 (1.04) | 81.4±12.2 (0.97) | 86.3±15.4 (1.03)a |
| Cigarette smoking (+/−)b | 20/46 | 18/15 (1.80)* | 9/28 (0.80) | 11/15 (1.40) |
| Alcohol consumption (+/−)c | 17/45 | 6/26 (0.68) | 4/29 (0.44) | 3/23 (0.42) |
| GLO1 genotype | ||||
| Wild type/mutant type | 59/8 | 25/8 (0.86) | 29/8 (0.89) | 24/2 (1.05) |
| Glu111Ala | 7 | 8 | 8 | 0 |
| Ala111Ala | 1 | 0 | 0 | 1 |
| Frameshift (T27NfsX15) | 0 | 0 | 0 | 1 |
| GLO1 enzymatic activity (mU/106 RBC) | 7.06±0.77 | 7.03±0.99 (1.00) | 6.84±0.88 (0.97)a | 6.32±0.97 (0.90)a,** |
| Clinical variables | ||||
| Acute exacerbation/chronic state | 0/67 | 2/31 (N/A) | 1/36 (N/A) | 0/26 (N/A) |
| Inpatients/outpatients | 16/51 | 13/20 (1.65) | 23/14 (2.60)** | 21/5 (3.38)*** |
| Family history (+/−) | 26/41 | 9/24 (0.70) | 16/21 (1.11) | 8/18 (0.79) |
| Education duration (y) | 13.0±2.6 | 13.3±2.3 (1.02) | 12.3±3.0 (0.94) | 11.7±2.6 (0.90)* |
| Onset (y) | 25.6±8.6 | 24.7±7.4 (0.96) | 24.1±8.0 (0.94) | 25.0±10.8 (0.98)a |
| Disease duration (y) | 21.3±14.1 | 23.5±15.0 (1.10) | 25.3±11.7 (1.19) | 26.4±15.5 (1.24)a |
| Number of hospitalizations | 3.0±3.0 | 3.1±2.8 (1.04) | 4.2±3.5 (1.39) | 4.5±4.8 (1.49) |
| Hospitalization duration (y) | 4.2±9.2 | 7.7±12.9 (1.85) | 8.7±10.2 (2.08)* | 17.4±16.9 (4.17)** |
| Antipsychotics (mg/day; CP equivalent) | 773.8±652.4 | 931.8±677.2 (1.20) | 1162.9±810.7 (1.50)* | 1143.9±743.6 (1.48)* |
| . | Normal Pentosidine . | High Pentosidine . | ||
|---|---|---|---|---|
| Normal Pyridoxal . | Low Pyridoxal . | Normal Pyridoxal . | Low Pyridoxal . | |
| Group 1 . | Group 2 (Fold Change) . | Group 3 (Fold Change) . | Group 4 (Fold Change) . | |
| Number of subjects | 67 | 33 | 37 | 26 |
| Demographics | ||||
| Schizoaffective/schizophrenia | 3/64 | 0/33 (N/A) | 1/36 (0.60) | 2/24 (1.72) |
| Pentosidine (ng/ml) | 41.5±11.6 | 39.4±10.8 (0.95) | 127.3±98.3 (3.07)*** | 123.0±85.6 (2.97)*** |
| Pyridoxal (ng/ml) | 9.5±5.7 | 3.4±1.1 (0.36)*** | 7.9±3.2 (0.83) | 3.4±1.0 (0.36)*** |
| Age | 46.9±14.7 | 48.2±12.6 (1.03) | 49.4±10.5 (1.05) | 51.4±11.7 (1.10) |
| Sex (male/female) | 28/39 | 26/7 (1.89)** | 16/21 (1.03) | 17/9 (1.56) |
| HbA1C | 5.0±0.3 | 5.0±0.3 (1.00) | 5.0±0.2 (1.00) | 5.0±0.4 (1.00)a |
| eGFR (ml/min) | 83.9±13.8 | 87.6±14.4 (1.04) | 81.4±12.2 (0.97) | 86.3±15.4 (1.03)a |
| Cigarette smoking (+/−)b | 20/46 | 18/15 (1.80)* | 9/28 (0.80) | 11/15 (1.40) |
| Alcohol consumption (+/−)c | 17/45 | 6/26 (0.68) | 4/29 (0.44) | 3/23 (0.42) |
| GLO1 genotype | ||||
| Wild type/mutant type | 59/8 | 25/8 (0.86) | 29/8 (0.89) | 24/2 (1.05) |
| Glu111Ala | 7 | 8 | 8 | 0 |
| Ala111Ala | 1 | 0 | 0 | 1 |
| Frameshift (T27NfsX15) | 0 | 0 | 0 | 1 |
| GLO1 enzymatic activity (mU/106 RBC) | 7.06±0.77 | 7.03±0.99 (1.00) | 6.84±0.88 (0.97)a | 6.32±0.97 (0.90)a,** |
| Clinical variables | ||||
| Acute exacerbation/chronic state | 0/67 | 2/31 (N/A) | 1/36 (N/A) | 0/26 (N/A) |
| Inpatients/outpatients | 16/51 | 13/20 (1.65) | 23/14 (2.60)** | 21/5 (3.38)*** |
| Family history (+/−) | 26/41 | 9/24 (0.70) | 16/21 (1.11) | 8/18 (0.79) |
| Education duration (y) | 13.0±2.6 | 13.3±2.3 (1.02) | 12.3±3.0 (0.94) | 11.7±2.6 (0.90)* |
| Onset (y) | 25.6±8.6 | 24.7±7.4 (0.96) | 24.1±8.0 (0.94) | 25.0±10.8 (0.98)a |
| Disease duration (y) | 21.3±14.1 | 23.5±15.0 (1.10) | 25.3±11.7 (1.19) | 26.4±15.5 (1.24)a |
| Number of hospitalizations | 3.0±3.0 | 3.1±2.8 (1.04) | 4.2±3.5 (1.39) | 4.5±4.8 (1.49) |
| Hospitalization duration (y) | 4.2±9.2 | 7.7±12.9 (1.85) | 8.7±10.2 (2.08)* | 17.4±16.9 (4.17)** |
| Antipsychotics (mg/day; CP equivalent) | 773.8±652.4 | 931.8±677.2 (1.20) | 1162.9±810.7 (1.50)* | 1143.9±743.6 (1.48)* |
Note: HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate; GLO1, glyoxalase 1; CP, chlorpromazine; N/A, not applicable. Cutoff point for high plasma pentosidine levels = 62.9ng/ml (the mean + 2 SDs of healthy controls). Low pyridoxal levels: <6ng/ml (male) and <4ng/ml (female). Fold change (FC) = relative rate (RR) defined in group 1 as 1.00. For categorical variables, FC was calculated as follows: RR in groups 2–4/RR in group 1.
We conducted unpaired t test in comparison of groups 2–4 with group 1, ANCOVA (vs group 1, covariate = age), and Fisher’s Exact test for categorical variables.
aANCOVA (vs group1, covariate = age).
bLack of data for 1 patient in group 1.
cLack of data for 5, 1, and 4 patients in groups 1, 2, and 3, respectively.
*P < .05 (vs group 1), **P < .001(vs group 1), ***P < .0001(vs group 1).
Measurement of Pentosidine and Vitamin B6
Fresh plasma and serum samples were obtained from all patients. Pentosidine was determined by high-performance liquid chromatography, as described previously.7 In brief, plasma samples were lyophilized and hydrolyzed in 100 µl of 6N hydrochloric acid for 16h, at 110°C under nitrogen. Samples were then neutralized with 100 µl of 5N sodium hydroxide and 200 µl of 0.5M sodium phosphate buffer (pH 7.4), filtered through a 0.5-µm filter, and finally diluted with phosphate- buffered saline. A sample corresponding to 25 µg of protein was injected into a high-performance liquid chromatography system and fractionated on a C18 reverse-phase column. Effluent was monitored at excitation-emission wavelengths of 335/385nm using a fluorescence detector (RF-10A; Shimadzu). Synthetic pentosidine was used to obtain a standard curve. The 3 forms of vitamin B6—pyridoxine, pyridoxal, and pyridoxamine—were measured from serum samples using high-performance liquid chromatography, according to a previously described method.8 Other parameters (glycohemoglobin AlC and creatinine,) were measured from blood samples. The GFR was estimated using the abbreviated Modification of Diet in Renal Diseases study equation.
Glyoxalase 1 Genotyping and Enzymatic Assay
We previously reported marked reductions (40%–50%) in glyoxalase 1 (GLO1) enzymatic activity in individuals carrying heterozygous frameshift mutations and that homozygous Ala111 carriers also exhibited significantly decreased enzymatic activity (an approximately 20% reduction) compared with homozygous Glu111 carriers in the schizophrenic group. In this study, we genotyped GLO1 mutations and measured GLO1 enzymatic activity using previously described methods6 to evaluate possible effects on plasma pentosidine levels.
Clinical Assessments
At the time of blood sample collection, we also assessed clinical variables such as inpatient or outpatient status, cigarette smoking status, alcohol consumption, family history, duration of education, onset of disease, durations of disease, and hospitalization, by conducting face-to-face interviews. This information was cross-referenced with medical records. The daily dose of antipsychotic medication was obtained from medical records and converted to chlorpromazine equivalents for each patient. The onset of disease was defined as the time point when clinical symptoms meeting DSM-IV diagnostic criteria first emerged. Having a family history of psychiatric disease was defined as having a first- or second-degree relative who had received any kind of psychiatric intervention. In the case of patients with multiple hospitalizations, the total duration of hospitalization was calculated as the sum total years of treatment in a psychiatric ward. Of the schizophrenic subjects, the symptom severity of 49 patients who agreed to being interviewed was assessed by PANSS. Assessors also ensured that these patients had no symptomatic exacerbation for at least 1 month prior to the interview. All participants provided written informed consent, and study protocols were approved by the ethics committees of all participating institutions (Tokyo Metropolitan Matsuzawa Hospital and Tokyo Metropolitan Institute of Medical Science).
Statistical Analysis
All data were analyzed using SPSS version 20.0 statistical software (IBM). Simple comparisons of means and standard errors of data between group 1 and the other groups were performed using the unpaired t test (both 2 tailed). In comparisons of HbA1c, eGFR, GLO1 enzymatic activity, onset of disease, and duration of disease between groups 1 and 4, we conducted ANCOVA as age was considered a covariate. Likewise, ANCOVA was also performed when comparing GLO1 enzymatic activity between groups 1 and 3. Fisher’s exact test was used for categorical variables and stepwise multiple regression analysis between psychological symptoms and each of the 2 measured biomarkers. Significance was defined as P < .05.
Results
Clinical Characterization
The mean (±SD) age of participants without carbonyl stress (group 1) and with enhanced carbonyl stress (group 4) were 46.9±14.7 and 51.4±11.7, respectively (P = .07). There were no significant differences linked to HbA1c, eGFR, smoking status, alcohol consumption, and male to female ratios in groups 1 and 4. In accordance with our prediction, the enhanced carbonyl stress group showed a more severe clinical course relative to the normal group (see table 1). The proportion of cases classified as inpatients were 23.9% (16 inpatients and 51 outpatients) in group 1 and 80.8% (21 inpatients and 5 outpatients) in group 4 (P < .0001). In addition, the mean (±SD) duration of hospitalization in group 1 was markedly shorter than that of group 4 (4.2±9.2 years vs 17.4±16.9 years, P = .0002). Furthermore, we noted higher daily doses of antipsychotics in group 4 compared with group 1 (773.8±652.4mg/day in group 1 and 1143.9±743.6mg/day in group 4, P = .02). We also observed lower educational status in group 4 compared with group 1 (P = .02). We were surprised to find that GLO1 enzymatic activity was significantly decreased in group 4, relative to group 1, despite group 4 containing a higher proportion of patients carrying the homozygous Glu111 allele compared with group 1 (P = .0005; see table 1). Significant differences in enzymatic activity remained, after excluding patients carrying frameshift mutations and homozygous Ala111 alleles from group 4. No significant differences were observed in other clinical parameters between groups 1 and 4. Intriguingly, groups 2 and 3 showed clinical features that were almost intermediate to those seen in groups 1 and 4 (see table 1).
Psychopathological Symptoms
Next, we evaluated clinical symptoms by analyzing the association between pentosidine and pyridoxal, biomarkers of enhanced carbonyl stress, and PANSS score (see table 2). We evaluated symptom severity of the 49 interviewed patients by PANSS. Of these 49 patients, 15 fell into group 1, 6 into group 2, 15 into group 3, and 13 into group 4. We conducted stepwise multiple regression analyses in which, age, in/outpatients status and antipsychotic medication were introduced into the model as confounding factors, as they are known to affect PANSS scores. We did not include other potential confound factors such as disease duration and hospitalizations because of high multicollinearity, defined as variance inflating factors, exceeding 2.0. Examining 4 subscales of PANSS, we found nominally significant, negative correlation between serum pyridoxal levels and items of the total positive symptom scale (standardized β = −.31, P = .0133), total general psychopathology score (standardized β = −.26, P = .0336), and the total PANSS score (standardized β = −.29, P = .0148). Pentosidine showed no association with PANSS scores (see table 2).
Association Between PANSS Score and Pentosidine and Pyridoxal Levels
| . | Clinical Variables . | Adjusted R2 . | Standardized β . | t Value . | P Value . |
|---|---|---|---|---|---|
| Total positive symptom score | Antipsychotics | .317 | .405 | 3.362 | .0016 |
| Pyridoxal | −.310 | −2.577 | .0133 | ||
| In/outpatients | .264 | 2.182 | .0344 | ||
| Total negative symptom score | Antipsychotics | .380 | .394 | 3.160 | .0028 |
| In/outpatients | .378 | 3.118 | .0032 | ||
| Age | .332 | 2.586 | .0130 | ||
| Total general psychopathology score | Antipsychotics | .384 | .481 | 3.867 | .0004 |
| Pyridoxal | −.259 | −2.194 | .0336 | ||
| In/outpatients | .259 | 2.146 | .0374 | ||
| Age | .224 | 1.694 | .0973 | ||
| Total PANSS score | Antipsychotics | .402 | .398 | 3.533 | .0010 |
| In/outpatients | .389 | 3.440 | .0013 | ||
| Pyridoxal | −.285 | −2.536 | .0148 |
| . | Clinical Variables . | Adjusted R2 . | Standardized β . | t Value . | P Value . |
|---|---|---|---|---|---|
| Total positive symptom score | Antipsychotics | .317 | .405 | 3.362 | .0016 |
| Pyridoxal | −.310 | −2.577 | .0133 | ||
| In/outpatients | .264 | 2.182 | .0344 | ||
| Total negative symptom score | Antipsychotics | .380 | .394 | 3.160 | .0028 |
| In/outpatients | .378 | 3.118 | .0032 | ||
| Age | .332 | 2.586 | .0130 | ||
| Total general psychopathology score | Antipsychotics | .384 | .481 | 3.867 | .0004 |
| Pyridoxal | −.259 | −2.194 | .0336 | ||
| In/outpatients | .259 | 2.146 | .0374 | ||
| Age | .224 | 1.694 | .0973 | ||
| Total PANSS score | Antipsychotics | .402 | .398 | 3.533 | .0010 |
| In/outpatients | .389 | 3.440 | .0013 | ||
| Pyridoxal | −.285 | −2.536 | .0148 |
Note: PANSS, Positive and Negative Syndrome Scale. Pentosidine, pyridoxal, age, antipsychotic medication, and in/outpatient status were introduced into the model as independent variables. Stepwise multiple regression analysis was performed. Corrected P values less than .0125 (.05/4) were considered statistically significant.
Association Between PANSS Score and Pentosidine and Pyridoxal Levels
| . | Clinical Variables . | Adjusted R2 . | Standardized β . | t Value . | P Value . |
|---|---|---|---|---|---|
| Total positive symptom score | Antipsychotics | .317 | .405 | 3.362 | .0016 |
| Pyridoxal | −.310 | −2.577 | .0133 | ||
| In/outpatients | .264 | 2.182 | .0344 | ||
| Total negative symptom score | Antipsychotics | .380 | .394 | 3.160 | .0028 |
| In/outpatients | .378 | 3.118 | .0032 | ||
| Age | .332 | 2.586 | .0130 | ||
| Total general psychopathology score | Antipsychotics | .384 | .481 | 3.867 | .0004 |
| Pyridoxal | −.259 | −2.194 | .0336 | ||
| In/outpatients | .259 | 2.146 | .0374 | ||
| Age | .224 | 1.694 | .0973 | ||
| Total PANSS score | Antipsychotics | .402 | .398 | 3.533 | .0010 |
| In/outpatients | .389 | 3.440 | .0013 | ||
| Pyridoxal | −.285 | −2.536 | .0148 |
| . | Clinical Variables . | Adjusted R2 . | Standardized β . | t Value . | P Value . |
|---|---|---|---|---|---|
| Total positive symptom score | Antipsychotics | .317 | .405 | 3.362 | .0016 |
| Pyridoxal | −.310 | −2.577 | .0133 | ||
| In/outpatients | .264 | 2.182 | .0344 | ||
| Total negative symptom score | Antipsychotics | .380 | .394 | 3.160 | .0028 |
| In/outpatients | .378 | 3.118 | .0032 | ||
| Age | .332 | 2.586 | .0130 | ||
| Total general psychopathology score | Antipsychotics | .384 | .481 | 3.867 | .0004 |
| Pyridoxal | −.259 | −2.194 | .0336 | ||
| In/outpatients | .259 | 2.146 | .0374 | ||
| Age | .224 | 1.694 | .0973 | ||
| Total PANSS score | Antipsychotics | .402 | .398 | 3.533 | .0010 |
| In/outpatients | .389 | 3.440 | .0013 | ||
| Pyridoxal | −.285 | −2.536 | .0148 |
Note: PANSS, Positive and Negative Syndrome Scale. Pentosidine, pyridoxal, age, antipsychotic medication, and in/outpatient status were introduced into the model as independent variables. Stepwise multiple regression analysis was performed. Corrected P values less than .0125 (.05/4) were considered statistically significant.
Discussion
This is the first study to focus on clinical features of schizophrenia observed in enhanced carbonyl stress. We found that a significantly higher proportion of subjects with carbonyl stress were inpatients, of low educational status, suffered longer durations of hospitalization, and were prescribed higher doses of antipsychotic medication, relative to subjects without carbonyl stress. Intriguingly, the 2 groups consisting of patients with either high pentosidine or low pyridoxal levels (group 2 or group 3) exhibited clinical features that were almost intermediate between groups 1 and 4. In addition, nominally significant negative association between serum pyridoxal levels and 3 subscales of PANSS—namely, the total positive symptom score, the total score for general psychopathology, and total PANSS scores—was observed. In contrast, plasma pentosidine levels showed no significant association with items of PANSS.
It should be noted that severe clinical features observed in patients with carbonyl stress, such as a higher inpatients status, a longer duration of hospitalization, and larger prescribed doses of antipsychotic medication, are very similar to those seen in treatment-resistant schizophrenia as defined by Kane et al.9 In this cohort of patients, clozapine is the standard treatment, proving more effective than conventional or other atypical antipsychotics.9–14 However, clozapine also induces serious and sometimes lethal adverse effects such as granulocytopenia.15,16 Intriguingly, psychopathological symptoms tended towards association with low pyridoxal levels. Although, the precise mechanisms of decreased pyridoxal levels in patients with enhanced carbonyl stress are not fully understood, our observations strongly support the theory that supplementation of vitamin B6 for these patients may safely improve specific clinical symptoms associated with pyridoxal levels. So far, a number of clinical reports, including 4 randomized placebo-controlled studies, have tested the efficacy of vitamin B6 supplementation for schizophrenia, but the results are inconsistent.17–24 A possible reason for the conflicting results could be that in these studies, no account was taken of the vitamin B6 levels in subjects. We believe that vitamin B6 supplementation may be most effective in schizophrenic patients with lower B6 levels associated with enhanced carbonyl stress. In terms of therapeutic benefits, this idea shares parallels with the use of clozapine in treatment-resistant schizophrenia. New clinical studies, utilizing vitamin B6 supplementation for schizophrenics with carbonyl stress are required.
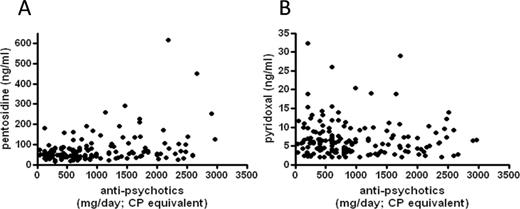
In this study, we cannot exclude possibility that high plasma pentosidine levels were a consequence of high doses and long time exposure of antipsychotic medications. In addition, it is well known that some second- generation antipsychotic drugs induce significantly greater weight gain than conventional antipsychotics.25,26 Indeed, simple regression models of statistical analysis revealed a significant positive correlation between plasma pentosidine and the daily dose of antipsychotic medication (Spearman’s correlation coefficient = 0.301, P < .0001; see figure 1). The lack of association with serum pyridoxal levels (Spearman’s correlation coefficient = −0.075, P = .34; see figure 1) appears to support this possibility. On the other hand, we have previously reported a case in which a drug-naive schizophrenic patient exhibited high plasma pentosidine.27 Furthermore, Cannon et al28 reported that DM in pregnant women increased the risk of schizophrenia in their offspring. These data endorse the hypothesis that exposure to enhanced carbonyl stress in the early stages of neural development may impact of physiological processes exemplified by the development of schizophrenia. Although we lacked information on environment factors, such as socioeconomic status of origin, for a number of subjects we put forward, the possibility that early exposure of high pentosidine may be causative of the low educational status observed in patients with enhanced carbonyl stress. Further studies focused on drug-naive patients will be required to address these issues.
Correlation between daily dose of antipsychotic drugs and pentosidine (A) and pyridoxal (B).
Psychopathological symptoms tended to correlate with serum pyridoxal, however, it remains unclear how decreased serum pyridoxal affects psychopathological symptoms. Pyridoxamine, one of the 3 forms of vitamin B6, is capable of scavenging pentosidine, and depletion of pyridoxamine during this process eventually leads to a decrease in serum pyridoxal levels. Given that pyridoxal is an important coenzyme in the synthesis of various neurotransmitters, such as dopamine, serotonin, and gamma-aminobutyric acid, the lack of pyridoxal in this situation raises the possibility that an imbalance of these neurotransmitters may influence severity of clinical features observed in these patients. Another possibility is that high pentosidine may itself affect clinical features by inducing an inflammatory response, through receptors for AGEs in the central nervous system (CNS). To examine this hypothesis, studies using animal models and human samples are necessary to reveal the precise molecular mechanisms of CNS involvement.
It was surprising to find that GLO1 activity was significantly decreased in group 4 compared with group 1 subjects, despite group 4 containing a higher proportion of homozygous Glu111 allele carriers (see table 1). The significant difference in activity between groups 1 and 4 persisted, even after excluding patients with frameshift mutations and homozygous Ala111 alleles. Although the exact mechanisms driving decreased GLO1 enzymatic activity in group 4 are unknown, preliminary data suggested that plasma zinc levels were significantly lower in schizophrenia relative to control samples (data not shown). So, one explanation for this paradoxical finding could be that enhanced carbonyl stress causes depletion of zinc ions, which are an essential trace element for maintaining GLO1 reactivity. In addition, other metabolic pathways, such as homocysteine metabolism, may be impaired under enhanced carbonyl stress conditions, because vitamin B6 plays a key role as a cofactor in this pathway. This lends plausible support to numerous recent reports investigating an association between elevated homocysteine levels and schizophrenia.21,29 Thus, we propose that enhanced carbonyl stress causes not only abnormalities in pentosidine and pyridoxal metabolism but also in wider metabolic pathways associated with these biomarkers.
As with studies of this nature, our study has some limitations. First, because this research is of a retrograde cross-sectional design, we cannot fully evaluate whether association between enhanced carbonyl stress and severe clinical course is a causal relationship. Future studies, implementing prospective and longitudinal designs, will be needed to elucidate the exact clinical relationship between this disease and the 2 proposed biomarkers. Second, as sample sizes for each group were relatively small, our analysis had limited power to detect statistical significance. This was particularly relevant in the PANSS analysis, where because interview data were available for only 49 patients, while we could detect trends, we were unable to detect significant differences by a direct comparison of PANSS scores between the groups (data not shown). Third, we did not control for other possible confound factors, including body mass index (BMI) and less severe disturbances of glucose metabolism. For BMI, we had limited data on group 1 subjects and data on all subjects, except 1, in group 4. We found that mean (±SD) BMI in the carbonyl stress group was 21.6±4.8 for males and 21.3±2.2 for females. These values are lower than the Japanese standard for people aged in their fifties (24.2 in males and 22.8 in females, http://www.stat.go.jp/data/nihon/21.htm). Therefore, we think that the influence of BMI on pentosidine or pyridoxal levels may be weak. Finally, because mild glucose intolerance and inflammation may possibly enhance carbonyl stress, studies into more sensitive markers, such as the homeostasis model assessment, which is an index of insulin resistance and C-reactive protein will be required in the future.
Conclusion
Patients with enhanced carbonyl stress showed distinct clinical features such as a higher propensity to inpatient status, low educational status, and longer durations of hospitalization, and higher doses of antipsychotic medication. These features bear strong similarity to those seen in treatment-resistant schizophrenia. It was also clear that psychopathological symptoms showed a tendency towards negative correlation with serum pyridoxal levels. Given that clozapine with its serious adverse effects is the most effective agent for treatment-resistant schizophrenia, our results support the idea that simple treatments that reduce carbonyl stress, such as supplementation of pyridoxamine, may be of novel therapeutic benefit for this subset of patients. As mentioned before, larger and longitudinal clinical studies will be required to validate these novel findings.
Funding
Japan Society for the Promotion of Science KAKENHI (23791368).
Acknowledgments
We especially thank Ms Hiroko Yuzawa at the Institute of Medical Sciences, Tokai University for the measurement of plasma pentosidine levels. We are also grateful for the expert technical assistance of Izumi Nohara, Mayumi Arai, and Nanako Obata. We appreciate Ms Yuuki Yoshida and Ms Ikuyo Kito who made technical advice and assisted with the preparation of the manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References