-
PDF
- Split View
-
Views
-
Cite
Cite
Karilynn M. Rockhill, Van T. Tong, Lucinda J. England, Denise V. D’Angelo, Nondaily Smokers’ Characteristics and Likelihood of Prenatal Cessation and Postpartum Relapse, Nicotine & Tobacco Research, Volume 19, Issue 7, 1 July 2017, Pages 810–816, https://doi.org/10.1093/ntr/ntw237
Close - Share Icon Share
Abstract
This study aimed to calculate the prevalence of pre-pregnancy nondaily smoking (<1 cigarette/day), risk factors, and report of prenatal provider smoking education; and assess the likelihood of prenatal cessation and postpartum relapse for nondaily smokers.
We analyzed data from 2009 to 2011 among women with live-born infants participating in the Pregnancy Risk Assessment Monitoring System. We compared characteristics of pre-pregnancy daily smokers (≥1 cigarette/day), nondaily smokers, and nonsmokers (chi-square adjusted p < .025). Between nondaily and daily smokers, we compared proportions of prenatal cessation, postpartum relapse (average 4 months postpartum), and reported provider education. Multivariable logistic regression calculated adjusted prevalence ratios (APR) for prenatal cessation among pre-pregnancy smokers (n = 27 360) and postpartum relapse among quitters (n = 13 577).
Nondaily smokers (11% of smokers) were more similar to nonsmokers and differed from daily smokers on characteristics examined (p ≤ .001 for all). Fewer nondaily smokers reported provider education than daily smokers (71.1%, 86.3%; p < .001). A higher proportion of nondaily compared to daily smokers quit during pregnancy (89.7%, 49.0%; p < .001), and a lower proportion relapsed postpartum (22.2%, 48.6%; p < .001). After adjustment, nondaily compared to daily smokers were more likely to quit (APR: 1.65; 95% confidence interval [CI]: 1.58–1.71) and less likely to relapse postpartum (APR: 0.55; 95% CI: 0.48–0.62).
Nondaily smokers were more likely to quit smoking during pregnancy, less likely to relapse postpartum, and less likely to report provider education than daily smokers. Providers should educate all women, regardless of frequency of use, about the harms of tobacco during pregnancy, provide effective cessation interventions, and encourage women to be tobacco free postpartum and beyond.
Nondaily smoking (<1 cigarette/day) is increasing among US smokers and carries a significant risk of disease. However, smoking patterns surrounding pregnancy among nondaily smokers are unknown. Using 2009–2011 data from the Pregnancy Risk Assessment Monitoring System, we found pre-pregnancy nondaily smokers compared to daily smokers were 65% more likely to quit smoking during pregnancy and almost half as likely to relapse postpartum. Providers should educate all women, regardless of frequency of use, about the harms of tobacco during pregnancy, provide effective cessation interventions, and encourage women to be tobacco free postpartum and beyond.
Introduction
“Nondaily” smoking includes a variety of behavioral smoking patterns with no standard definition.1 It has been described in the literature as intermittent, occasional, social, and “some-day” smoking.1 Nondaily smokers are a heterogeneous group with behaviors characterized by a range of practices, such as only smoking in social situations, displaying stable smoking patterns, recently converting from daily smoking, or exhibiting a wide variation in the amount of cigarettes consumed per smoking episode.1,2 Although the overall smoking prevalence in US adults has decreased, the prevalence of nondaily smoking is increasing among current smokers, rising from an estimated 19.2% (8.7 million persons) in 2005 to 23.1% (9.7 million persons) in 2013.3 The highest proportions of nondaily smoking are found among minorities, young adults, and females.1,2,4 Despite the possibility of overall less tobacco exposure than daily smokers, nondaily smoking still carries substantial long-term health risks. Nondaily and low-intensity smokers still have a higher lifetime risk of cardiovascular disease, and a higher risk of lung and other cancers than nonsmokers.1,5 Among females, nondaily and low intensity smokers have a 4- to 6-year median loss of life compared to nonsmokers.1
All levels of prenatal tobacco exposure, ranging from active smoking to exposure to secondhand smoke, can reduce infant birth weight and increase the risk of preterm deliveries.6,7 Previous studies have examined patterns of use during pregnancy among daily smokers and rates of quitting and postpartum relapse.8,9 The prevalence of nondaily smoking during pregnancy and the prevalence of provider education about the harms of smoking during pregnancy to this group are unknown. In addition, nondaily smoking has been associated with other risky behaviors, such as drinking alcohol, in both pregnant and nonpregnant women.1,10–12 There is evidence of a possible synergistic relationship between concurrent use of tobacco and alcohol during pregnancy and adverse perinatal outcomes such as fetal growth restriction, preterm birth, low birth weight, and congenital cardiac defects.13,14
The Pregnancy Risk Assessment Monitoring System (PRAMS) is a surveillance system of perinatal behaviors which can be used to examine respondents’ report of nondaily smoking (<1 cigarette/day) and has been shown to identify more smokers than using smoking measures reported the birth certificate.15 The objectives of this study were to: (1) evaluate the prevalence and risk factors associated with nondaily smoking, and (2) assess the likelihood of prenatal quitting and postpartum relapse in nondaily smokers compared to daily smokers and examine receipt of provider education during prenatal care about harms of smoking.
Methods
Data Source
PRAMS is an annual survey of women who have recently delivered a live born infant, which assesses behaviors and experiences before, during, and after pregnancy. Women are sampled monthly from birth certificate records and are sent a mail survey within 2 and 6 months postpartum. Women who do not respond to the first mailing, receive up to two more mail surveys followed by a telephone interview. PRAMS survey responses are linked to the infant’s birth certificate. More details about the PRAMS methodology can be found elsewhere.16 For this analysis, data were aggregated from 31 states (Alaska, Arkansas, Colorado, Delaware, Georgia, Hawaii, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New Jersey, New Mexico, New York State, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, Tennessee, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming) and New York City, which participated in PRAMS 1 or more years during 2009 through 2011. Each state was included for years in which they met a ≥65% response rate threshold, and data were weighted to adjust for sample design, noncoverage, and nonresponse. The weighted sample is representative of approximately 46% of US births during 2009–2011.
Variables
On the PRAMS questionnaire, women were asked if they had smoked any cigarettes in the previous 2 years. Nonsmokers were defined as women who reported “no” to smoking any cigarettes in the previous 2 years. Women who responded “yes” were then asked for the average number of cigarettes smoked per day in the 3 months before pregnancy, in the last 3 months of pregnancy, and at the time of survey administration (postpartum). Categorical response options for daily smoking intensity during all three time periods were <1, 1 to 5, 6 to 10, 11 to 20, 21 to 40, or 41 or more cigarette(s) per day. Nondaily smokers were defined as women reporting smoking an average of <1 cigarette per day in the 3 months before pregnancy. Daily smokers were defined as women reporting smoking 1–41 or more cigarettes per day in the 3 months before pregnancy. Smoking cessation was defined as any smoking in the 3 months before pregnancy, but no smoking during the last 3 months of pregnancy (ie, quitting during pregnancy). Postpartum relapse was defined as any smoking at the time of the postpartum survey among women who had quit smoking during pregnancy. Maternal age, household annual income, pre-pregnancy body mass index (BMI), pregnancy intention, health insurance coverage before pregnancy, any alcohol use, and any binge drinking (four or more drinks in one sitting) were all based on responses in the PRAMS questionnaire; maternal race/ethnicity, education level, marital status, and parity were obtained from linked birth certificate data.
Provider education during prenatal care about the harms of smoking was assessed from the PRAMS question: “During any of your prenatal care visits, did a doctor, nurse, or other health care worker talk with you about any of the things listed below? Please count only discussions, not reading materials or videos.” The option of interest read, “How smoking during pregnancy could affect my baby.”
Analysis
The percentages and 95% confidence intervals (CIs) of selected demographic and pregnancy-related characteristics were calculated by smoking status. Characteristics of nondaily smokers were compared to both nonsmokers and daily smokers separately using chi-square tests and Bonferroni adjusted p values for statistical significance (p < .025). In a post hoc analysis due to the differences observed for alcohol use and binge drinking before pregnancy by smoking status, the proportions of women who reported alcohol use and binge drinking during the last 3 months of pregnancy were also described using similar chi-square tests and adjusted p values (p < .025).
The transitional smoking status among pre-pregnancy smokers from the 3 months before pregnancy to last 3 months of pregnancy were calculated by pre-pregnancy smoking intensity category, and then the prevalence and 95% CIs of smoking cessation, postpartum relapse, and reported provider education during prenatal care were calculated; differences between nondaily and daily smokers were assessed using chi-square tests. Two multivariable regression models were conducted to calculate adjusted prevalence ratios (APR) using predicted marginal risks for independent associations with smoking status controlling for potential confounders.17 In the first model, smoking cessation during pregnancy was examined among all pre-pregnancy smokers (n = 27 360), controlling for maternal age, race/ethnicity, education level, marital status, pregnancy intention, parity, pre-pregnancy insurance coverage, pre-pregnancy alcohol use, state, and infant birth year. In the second model, postpartum relapse was examined among all women who quit smoking during pregnancy (n = 13 577), controlling for maternal age, race/ethnicity, education level, marital status, pregnancy intention, parity, pre-pregnancy alcohol use, state, and infant birth year. For both models, confounders were chosen based on potential causal factors from the literature and crude associations between the exposure and outcomes with the covariates (p < .05).9,18 Women with missing information on one or more of the covariates in the regression models were excluded, which was 8% of the sample for smoking cessation and 5% of the sample for postpartum relapse. However, data from excluded women did not differ from that of included women by smoking status or by outcome.
All data analyses were conducted in 2015 using SAS version 9.3 (Cary, NC) and SUDAAN version 11 (Research Triangle, NC) to account for the complex survey design of PRAMS. The Centers for Disease Control Institutional Review Board approved the PRAMS protocol, and all participating states and New York City approved the study analysis plan. A p value of <.05 was considered statistically significant unless otherwise indicated.
Results
The PRAMS survey was administered an average of 118 days (SD = 34 days, range = 61–270 days), or about 4 months, after delivery. Among the weighted sample of women, 76% were nonsmokers, 3% were nondaily smokers, and 21% were daily smokers in the 3 months before pregnancy. Nondaily smokers accounted for 11% of all smokers.
Nondaily smokers had more characteristics that were similar to those of nonsmokers than were similar to daily smokers as shown in Table 1. Nondaily smokers and nonsmokers were not significantly different with respect to annual income, pre-pregnancy BMI, and health insurance status. Compared to daily smokers, both nondaily smokers and nonsmokers had higher proportions of women who were Hispanic, had >12 years of education, had ≥$15 000 annual income, were normal weight, and were on private insurance before pregnancy. In contrast, nondaily smokers and daily smokers were significantly different across all risk factors examined (p ≤ .001 for all). Compared to daily smokers, a lower proportion of nondaily smokers had unintended pregnancies, had higher order births, and were unmarried. By contrast, nondaily smokers had significantly higher proportions of women who used any alcohol and binge drank in the 3 months before pregnancy compared to both nonsmokers and daily smokers (p < .001 for both). Based on these findings, we further examined whether nondaily smoking and daily smoking were associated with alcohol use during the last 3 months of pregnancy. Any alcohol use in the last 3 months of pregnancy was significantly higher for nondaily smokers (12.9%) compared to both nonsmokers (7.0%) and daily smokers (6.3%; p < .001). Report of binge drinking during the last 3 months of pregnancy was not significantly different between the smoking categories: 0.8% in nonsmokers, 1.1% in nondaily smokers, and 1.0% in daily smokers.
Demographic and Pregnancy-Related Characteristics of Women by Smoking Status in the 3 Months Before Pregnancya
| . | Smoking status before pregnancyb . | p value for nondaily smokersc . | |||
|---|---|---|---|---|---|
| . | Nonsmokers . | Nondaily smokers . | Daily smokers . | vs. Nonsmokers . | vs. Daily smokers . |
| Characteristics . | % (95% CI) . | % (95% CI) . | % (95% CI) . | ||
| Maternal age (y) | <.001 | <.001 | |||
| <20 | 8.1 (7.8–8.5) | 9.4 (7.6–11.6) | 12.9 (12.1–13.7) | ||
| 20–24 | 19.4 (18.9–19.9) | 26.2 (23.3–29.2) | 34.1 (33.0–35.1) | ||
| 25–34 | 56.3 (55.7–56.9) | 54.0 (50.7–57.1) | 45.6 (44.5–46.7) | ||
| ≥35 | 16.2 (15.7–16.6) | 10.5 (8.7–12.6) | 7.5 (6.9–8.0) | ||
| Maternal race/ethnicity | <.001 | <.001 | |||
| White, non-Hispanic | 54.4 (53.9–54.8) | 65.5 (62.3–68.7) | 73.0 (72.0–73.9) | ||
| Black, non-Hispanic | 14.0 (13.6–14.3) | 6.5 (5.3–7.8) | 12.2 (11.6–12.9) | ||
| Hispanic | 23.1 (22.7–23.4) | 20.9 (17.9–24.2) | 9.0 (8.3–9.8) | ||
| Other race/ethnicity | 8.6 (8.3–8.9) | 7.1 (5.7–8.7) | 5.8 (5.4–6.2) | ||
| Maternal education (y) | .009 | <.001 | |||
| <12 | 16.3 (15.9–16.8) | 12.6 (10.3–15.3) | 22.2 (21.3–23.1) | ||
| 12 | 22.6 (22.1–23.2) | 22.6 (19.9–25.5) | 39.3 (38.2–40.4) | ||
| >12 | 61.0 (60.5–61.6) | 64.8 (61.6–68.0) | 38.5 (37.5–39.6) | ||
| Marital status | <.001 | <.001 | |||
| Unmarried | 32.8 (32.2–33.3) | 40.6 (37.5–43.9) | 63.2 (62.2–64.2) | ||
| Married | 67.2 (66.7–67.8) | 59.4 (56.1–62.5) | 36.8 (35.8–37.8) | ||
| Annual income | .353 | <.001 | |||
| <$15 000 | 26.2 (25.7–26.8) | 24.8 (22.0–27.8) | 46.5 (45.4–47.7) | ||
| ≥$15 000 | 73.8 (73.2–74.3) | 75.2 (72.2–78.0) | 53.5 (52.3–54.6) | ||
| Pre-pregnancy body mass index (kg/m2) | .974 | .001 | |||
| Underweight (<18.5) | 4.0 (3.7–4.2) | 4.1 (3.0–5.5) | 5.6 (5.1–6.1) | ||
| Normal weight (18.5–24.9) | 51.7 (51.1–52.3) | 51.5 (48.2–54.7) | 45.5 (44.4–46.6) | ||
| Overweight (25.0–29.9) | 24.2 (23.6–24.7) | 23.7 (21.1–26.5) | 24.1 (23.2–25.1) | ||
| Obese (≥30.0) | 20.2 (19.7–20.7) | 20.7 (18.1–23.6) | 24.8 (23.8–25.7) | ||
| Pregnancy intention | <.001 | <.001 | |||
| Intended | 62.1 (61.5–62.7) | 53.2 (49.9–56.4) | 42.8 (41.7–43.9) | ||
| Unintended | 37.9 (37.3–38.5) | 46.8 (43.6–50.1) | 57.2 (56.1–58.3) | ||
| Parity | <.001 | <.001 | |||
| First birth | 40.9 (40.3–41.5) | 52.3 (49.1–55.5) | 44.8 (43.7–45.9) | ||
| Second or later birth | 59.1 (58.5–59.7) | 47.7 (44.5–50.9) | 55.2 (54.1–56.3) | ||
| Health insurance coverage before pregnancy | .056 | <.001 | |||
| Private | 61.1 (60.5–61.7) | 63.0 (59.8–66.2) | 38.0 (36.9–39.1) | ||
| Medicaid | 14.3 (13.9–14.7) | 12.0 (10.2–14.1) | 27.5 (26.6–28.5) | ||
| Other insuranced | 2.2 (2.1–2.4) | 1.7 (1.2–2.4) | 3.0 (2.7–3.3) | ||
| Uninsured | 22.4 (21.9–22.9) | 23.3 (20.4–26.3) | 31.5 (30.4–32.5) | ||
| Alcohol use in 3 months before pregnancy | <.001 | <.001 | |||
| Yes | 46.9 (46.3–47.5) | 87.1 (84.6–89.3) | 71.5 (70.5–72.5) | ||
| Binge drinking in 3 months before pregnancye | <.001 | <.001 | |||
| Yes | 16.9 (16.4–17.3) | 56.1 (52.8–59.2) | 42.7 (41.6–43.8) | ||
| . | Smoking status before pregnancyb . | p value for nondaily smokersc . | |||
|---|---|---|---|---|---|
| . | Nonsmokers . | Nondaily smokers . | Daily smokers . | vs. Nonsmokers . | vs. Daily smokers . |
| Characteristics . | % (95% CI) . | % (95% CI) . | % (95% CI) . | ||
| Maternal age (y) | <.001 | <.001 | |||
| <20 | 8.1 (7.8–8.5) | 9.4 (7.6–11.6) | 12.9 (12.1–13.7) | ||
| 20–24 | 19.4 (18.9–19.9) | 26.2 (23.3–29.2) | 34.1 (33.0–35.1) | ||
| 25–34 | 56.3 (55.7–56.9) | 54.0 (50.7–57.1) | 45.6 (44.5–46.7) | ||
| ≥35 | 16.2 (15.7–16.6) | 10.5 (8.7–12.6) | 7.5 (6.9–8.0) | ||
| Maternal race/ethnicity | <.001 | <.001 | |||
| White, non-Hispanic | 54.4 (53.9–54.8) | 65.5 (62.3–68.7) | 73.0 (72.0–73.9) | ||
| Black, non-Hispanic | 14.0 (13.6–14.3) | 6.5 (5.3–7.8) | 12.2 (11.6–12.9) | ||
| Hispanic | 23.1 (22.7–23.4) | 20.9 (17.9–24.2) | 9.0 (8.3–9.8) | ||
| Other race/ethnicity | 8.6 (8.3–8.9) | 7.1 (5.7–8.7) | 5.8 (5.4–6.2) | ||
| Maternal education (y) | .009 | <.001 | |||
| <12 | 16.3 (15.9–16.8) | 12.6 (10.3–15.3) | 22.2 (21.3–23.1) | ||
| 12 | 22.6 (22.1–23.2) | 22.6 (19.9–25.5) | 39.3 (38.2–40.4) | ||
| >12 | 61.0 (60.5–61.6) | 64.8 (61.6–68.0) | 38.5 (37.5–39.6) | ||
| Marital status | <.001 | <.001 | |||
| Unmarried | 32.8 (32.2–33.3) | 40.6 (37.5–43.9) | 63.2 (62.2–64.2) | ||
| Married | 67.2 (66.7–67.8) | 59.4 (56.1–62.5) | 36.8 (35.8–37.8) | ||
| Annual income | .353 | <.001 | |||
| <$15 000 | 26.2 (25.7–26.8) | 24.8 (22.0–27.8) | 46.5 (45.4–47.7) | ||
| ≥$15 000 | 73.8 (73.2–74.3) | 75.2 (72.2–78.0) | 53.5 (52.3–54.6) | ||
| Pre-pregnancy body mass index (kg/m2) | .974 | .001 | |||
| Underweight (<18.5) | 4.0 (3.7–4.2) | 4.1 (3.0–5.5) | 5.6 (5.1–6.1) | ||
| Normal weight (18.5–24.9) | 51.7 (51.1–52.3) | 51.5 (48.2–54.7) | 45.5 (44.4–46.6) | ||
| Overweight (25.0–29.9) | 24.2 (23.6–24.7) | 23.7 (21.1–26.5) | 24.1 (23.2–25.1) | ||
| Obese (≥30.0) | 20.2 (19.7–20.7) | 20.7 (18.1–23.6) | 24.8 (23.8–25.7) | ||
| Pregnancy intention | <.001 | <.001 | |||
| Intended | 62.1 (61.5–62.7) | 53.2 (49.9–56.4) | 42.8 (41.7–43.9) | ||
| Unintended | 37.9 (37.3–38.5) | 46.8 (43.6–50.1) | 57.2 (56.1–58.3) | ||
| Parity | <.001 | <.001 | |||
| First birth | 40.9 (40.3–41.5) | 52.3 (49.1–55.5) | 44.8 (43.7–45.9) | ||
| Second or later birth | 59.1 (58.5–59.7) | 47.7 (44.5–50.9) | 55.2 (54.1–56.3) | ||
| Health insurance coverage before pregnancy | .056 | <.001 | |||
| Private | 61.1 (60.5–61.7) | 63.0 (59.8–66.2) | 38.0 (36.9–39.1) | ||
| Medicaid | 14.3 (13.9–14.7) | 12.0 (10.2–14.1) | 27.5 (26.6–28.5) | ||
| Other insuranced | 2.2 (2.1–2.4) | 1.7 (1.2–2.4) | 3.0 (2.7–3.3) | ||
| Uninsured | 22.4 (21.9–22.9) | 23.3 (20.4–26.3) | 31.5 (30.4–32.5) | ||
| Alcohol use in 3 months before pregnancy | <.001 | <.001 | |||
| Yes | 46.9 (46.3–47.5) | 87.1 (84.6–89.3) | 71.5 (70.5–72.5) | ||
| Binge drinking in 3 months before pregnancye | <.001 | <.001 | |||
| Yes | 16.9 (16.4–17.3) | 56.1 (52.8–59.2) | 42.7 (41.6–43.8) | ||
CI = confidence interval.
aIncluded 31 Pregnancy Risk Assessment Monitoring System states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011.
bSelf-report smoking status in the 3 months before pregnancy: Nondaily smokers, average <1 cigarette/day; Daily smokers, average ≥1 cigarette(s)/day.
cBonferroni adjusted p value for dual comparisons (p < .025).
dOther health insurance coverage includes Tricare, other military health insurance, Indian Health Service, or state-specific State Children’s Health Insurance Program.
eBinge drinking defined as four or more drinks in one sitting during the 3 months before pregnancy.
Demographic and Pregnancy-Related Characteristics of Women by Smoking Status in the 3 Months Before Pregnancya
| . | Smoking status before pregnancyb . | p value for nondaily smokersc . | |||
|---|---|---|---|---|---|
| . | Nonsmokers . | Nondaily smokers . | Daily smokers . | vs. Nonsmokers . | vs. Daily smokers . |
| Characteristics . | % (95% CI) . | % (95% CI) . | % (95% CI) . | ||
| Maternal age (y) | <.001 | <.001 | |||
| <20 | 8.1 (7.8–8.5) | 9.4 (7.6–11.6) | 12.9 (12.1–13.7) | ||
| 20–24 | 19.4 (18.9–19.9) | 26.2 (23.3–29.2) | 34.1 (33.0–35.1) | ||
| 25–34 | 56.3 (55.7–56.9) | 54.0 (50.7–57.1) | 45.6 (44.5–46.7) | ||
| ≥35 | 16.2 (15.7–16.6) | 10.5 (8.7–12.6) | 7.5 (6.9–8.0) | ||
| Maternal race/ethnicity | <.001 | <.001 | |||
| White, non-Hispanic | 54.4 (53.9–54.8) | 65.5 (62.3–68.7) | 73.0 (72.0–73.9) | ||
| Black, non-Hispanic | 14.0 (13.6–14.3) | 6.5 (5.3–7.8) | 12.2 (11.6–12.9) | ||
| Hispanic | 23.1 (22.7–23.4) | 20.9 (17.9–24.2) | 9.0 (8.3–9.8) | ||
| Other race/ethnicity | 8.6 (8.3–8.9) | 7.1 (5.7–8.7) | 5.8 (5.4–6.2) | ||
| Maternal education (y) | .009 | <.001 | |||
| <12 | 16.3 (15.9–16.8) | 12.6 (10.3–15.3) | 22.2 (21.3–23.1) | ||
| 12 | 22.6 (22.1–23.2) | 22.6 (19.9–25.5) | 39.3 (38.2–40.4) | ||
| >12 | 61.0 (60.5–61.6) | 64.8 (61.6–68.0) | 38.5 (37.5–39.6) | ||
| Marital status | <.001 | <.001 | |||
| Unmarried | 32.8 (32.2–33.3) | 40.6 (37.5–43.9) | 63.2 (62.2–64.2) | ||
| Married | 67.2 (66.7–67.8) | 59.4 (56.1–62.5) | 36.8 (35.8–37.8) | ||
| Annual income | .353 | <.001 | |||
| <$15 000 | 26.2 (25.7–26.8) | 24.8 (22.0–27.8) | 46.5 (45.4–47.7) | ||
| ≥$15 000 | 73.8 (73.2–74.3) | 75.2 (72.2–78.0) | 53.5 (52.3–54.6) | ||
| Pre-pregnancy body mass index (kg/m2) | .974 | .001 | |||
| Underweight (<18.5) | 4.0 (3.7–4.2) | 4.1 (3.0–5.5) | 5.6 (5.1–6.1) | ||
| Normal weight (18.5–24.9) | 51.7 (51.1–52.3) | 51.5 (48.2–54.7) | 45.5 (44.4–46.6) | ||
| Overweight (25.0–29.9) | 24.2 (23.6–24.7) | 23.7 (21.1–26.5) | 24.1 (23.2–25.1) | ||
| Obese (≥30.0) | 20.2 (19.7–20.7) | 20.7 (18.1–23.6) | 24.8 (23.8–25.7) | ||
| Pregnancy intention | <.001 | <.001 | |||
| Intended | 62.1 (61.5–62.7) | 53.2 (49.9–56.4) | 42.8 (41.7–43.9) | ||
| Unintended | 37.9 (37.3–38.5) | 46.8 (43.6–50.1) | 57.2 (56.1–58.3) | ||
| Parity | <.001 | <.001 | |||
| First birth | 40.9 (40.3–41.5) | 52.3 (49.1–55.5) | 44.8 (43.7–45.9) | ||
| Second or later birth | 59.1 (58.5–59.7) | 47.7 (44.5–50.9) | 55.2 (54.1–56.3) | ||
| Health insurance coverage before pregnancy | .056 | <.001 | |||
| Private | 61.1 (60.5–61.7) | 63.0 (59.8–66.2) | 38.0 (36.9–39.1) | ||
| Medicaid | 14.3 (13.9–14.7) | 12.0 (10.2–14.1) | 27.5 (26.6–28.5) | ||
| Other insuranced | 2.2 (2.1–2.4) | 1.7 (1.2–2.4) | 3.0 (2.7–3.3) | ||
| Uninsured | 22.4 (21.9–22.9) | 23.3 (20.4–26.3) | 31.5 (30.4–32.5) | ||
| Alcohol use in 3 months before pregnancy | <.001 | <.001 | |||
| Yes | 46.9 (46.3–47.5) | 87.1 (84.6–89.3) | 71.5 (70.5–72.5) | ||
| Binge drinking in 3 months before pregnancye | <.001 | <.001 | |||
| Yes | 16.9 (16.4–17.3) | 56.1 (52.8–59.2) | 42.7 (41.6–43.8) | ||
| . | Smoking status before pregnancyb . | p value for nondaily smokersc . | |||
|---|---|---|---|---|---|
| . | Nonsmokers . | Nondaily smokers . | Daily smokers . | vs. Nonsmokers . | vs. Daily smokers . |
| Characteristics . | % (95% CI) . | % (95% CI) . | % (95% CI) . | ||
| Maternal age (y) | <.001 | <.001 | |||
| <20 | 8.1 (7.8–8.5) | 9.4 (7.6–11.6) | 12.9 (12.1–13.7) | ||
| 20–24 | 19.4 (18.9–19.9) | 26.2 (23.3–29.2) | 34.1 (33.0–35.1) | ||
| 25–34 | 56.3 (55.7–56.9) | 54.0 (50.7–57.1) | 45.6 (44.5–46.7) | ||
| ≥35 | 16.2 (15.7–16.6) | 10.5 (8.7–12.6) | 7.5 (6.9–8.0) | ||
| Maternal race/ethnicity | <.001 | <.001 | |||
| White, non-Hispanic | 54.4 (53.9–54.8) | 65.5 (62.3–68.7) | 73.0 (72.0–73.9) | ||
| Black, non-Hispanic | 14.0 (13.6–14.3) | 6.5 (5.3–7.8) | 12.2 (11.6–12.9) | ||
| Hispanic | 23.1 (22.7–23.4) | 20.9 (17.9–24.2) | 9.0 (8.3–9.8) | ||
| Other race/ethnicity | 8.6 (8.3–8.9) | 7.1 (5.7–8.7) | 5.8 (5.4–6.2) | ||
| Maternal education (y) | .009 | <.001 | |||
| <12 | 16.3 (15.9–16.8) | 12.6 (10.3–15.3) | 22.2 (21.3–23.1) | ||
| 12 | 22.6 (22.1–23.2) | 22.6 (19.9–25.5) | 39.3 (38.2–40.4) | ||
| >12 | 61.0 (60.5–61.6) | 64.8 (61.6–68.0) | 38.5 (37.5–39.6) | ||
| Marital status | <.001 | <.001 | |||
| Unmarried | 32.8 (32.2–33.3) | 40.6 (37.5–43.9) | 63.2 (62.2–64.2) | ||
| Married | 67.2 (66.7–67.8) | 59.4 (56.1–62.5) | 36.8 (35.8–37.8) | ||
| Annual income | .353 | <.001 | |||
| <$15 000 | 26.2 (25.7–26.8) | 24.8 (22.0–27.8) | 46.5 (45.4–47.7) | ||
| ≥$15 000 | 73.8 (73.2–74.3) | 75.2 (72.2–78.0) | 53.5 (52.3–54.6) | ||
| Pre-pregnancy body mass index (kg/m2) | .974 | .001 | |||
| Underweight (<18.5) | 4.0 (3.7–4.2) | 4.1 (3.0–5.5) | 5.6 (5.1–6.1) | ||
| Normal weight (18.5–24.9) | 51.7 (51.1–52.3) | 51.5 (48.2–54.7) | 45.5 (44.4–46.6) | ||
| Overweight (25.0–29.9) | 24.2 (23.6–24.7) | 23.7 (21.1–26.5) | 24.1 (23.2–25.1) | ||
| Obese (≥30.0) | 20.2 (19.7–20.7) | 20.7 (18.1–23.6) | 24.8 (23.8–25.7) | ||
| Pregnancy intention | <.001 | <.001 | |||
| Intended | 62.1 (61.5–62.7) | 53.2 (49.9–56.4) | 42.8 (41.7–43.9) | ||
| Unintended | 37.9 (37.3–38.5) | 46.8 (43.6–50.1) | 57.2 (56.1–58.3) | ||
| Parity | <.001 | <.001 | |||
| First birth | 40.9 (40.3–41.5) | 52.3 (49.1–55.5) | 44.8 (43.7–45.9) | ||
| Second or later birth | 59.1 (58.5–59.7) | 47.7 (44.5–50.9) | 55.2 (54.1–56.3) | ||
| Health insurance coverage before pregnancy | .056 | <.001 | |||
| Private | 61.1 (60.5–61.7) | 63.0 (59.8–66.2) | 38.0 (36.9–39.1) | ||
| Medicaid | 14.3 (13.9–14.7) | 12.0 (10.2–14.1) | 27.5 (26.6–28.5) | ||
| Other insuranced | 2.2 (2.1–2.4) | 1.7 (1.2–2.4) | 3.0 (2.7–3.3) | ||
| Uninsured | 22.4 (21.9–22.9) | 23.3 (20.4–26.3) | 31.5 (30.4–32.5) | ||
| Alcohol use in 3 months before pregnancy | <.001 | <.001 | |||
| Yes | 46.9 (46.3–47.5) | 87.1 (84.6–89.3) | 71.5 (70.5–72.5) | ||
| Binge drinking in 3 months before pregnancye | <.001 | <.001 | |||
| Yes | 16.9 (16.4–17.3) | 56.1 (52.8–59.2) | 42.7 (41.6–43.8) | ||
CI = confidence interval.
aIncluded 31 Pregnancy Risk Assessment Monitoring System states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011.
bSelf-report smoking status in the 3 months before pregnancy: Nondaily smokers, average <1 cigarette/day; Daily smokers, average ≥1 cigarette(s)/day.
cBonferroni adjusted p value for dual comparisons (p < .025).
dOther health insurance coverage includes Tricare, other military health insurance, Indian Health Service, or state-specific State Children’s Health Insurance Program.
eBinge drinking defined as four or more drinks in one sitting during the 3 months before pregnancy.
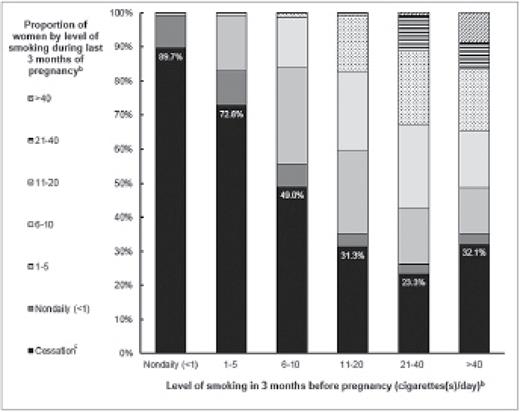
Among pre-pregnancy nondaily smokers, 89.7% reported no cigarette use in the last 3 months of pregnancy, 9.4% reported continuing nondaily smoking, and <1% reported increasing to daily smoking graphed in Figure 1. The proportion of women who reported cessation was significantly higher among nondaily smokers than among all categories of daily smoking, including those smoking 1–5 cigarettes/day (p < .001). The proportion of daily smokers who reported cessation differed by pre-pregnancy cigarette consumption, with the lowest proportion among 21–40 cigarettes/day smokers (23.3%) and the highest proportion among 1–5 cigarettes/day smokers (72.8%) displayed in Figure 1 and described in Table 2. After controlling for potential confounders, nondaily smokers were 1.65 times more likely to quit smoking by the last 3 months of pregnancy than daily smokers (APR = 1.65; 95% CI: 1.58–1.71).
Transitional smoking status during pregnancy.a The transitional smoking status among all smokers between the 3 months before pregnancy to the last 3 months of pregnancy by pre-pregnancy smoking intensity with the proportion of smoking cessation highlighted. aIncluded in analysis was 31 Pregnancy Risk Assessment Monitoring System (PRAMS) states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011. bCategories of maternal report of average cigarette(s)/day on PRAMS survey. cPrenatal smoking cessation was defined as smoking in the 3 months before pregnancy, but not smoking during the last 3 months of pregnancy (ie, quitting).
Proportion of Women Who Reported Prenatal Smoking Cessation and Postpartum Relapse and Adjusted Prevalence Ratios (APRs)a
| Smoking status in 3 months before pregnancy . | Proportion of prenatal smoking cessationb . | Proportion of postpartum smoking relapsec . | ||
|---|---|---|---|---|
| % (95% CI)d . | APRe . | % (95% CI)f . | APRg . | |
| Nondaily smokers (<1 cigarette/day) | 89.7 (87.7–94.4)h | 1.65 (1.58–1.71) | 22.2 (19.5–25.1)h | 0.55 (0.48–0.62) |
| Daily smokers (cigarette(s)/day) | 49.0 (47.9–50.1) | reference | 48.6 (47.0–50.2) | reference |
| 1–5 | 72.8 (71.1–74.5) | 45.3 (43.0–47.7) | ||
| 6–10 | 49.0 (47.0–50.9) | 52.7 (49.8–55.6) | ||
| 11–20 | 31.3 (29.5–33.2) | 49.5 (45.9–53.1) | ||
| 21–40 | 23.3 (19.9–27.1) | 51.5 (42.6–60.3) | ||
| >40 | 32.1 (24.0–41.5) | 53.2 (36.6–69.1) | ||
| Smoking status in 3 months before pregnancy . | Proportion of prenatal smoking cessationb . | Proportion of postpartum smoking relapsec . | ||
|---|---|---|---|---|
| % (95% CI)d . | APRe . | % (95% CI)f . | APRg . | |
| Nondaily smokers (<1 cigarette/day) | 89.7 (87.7–94.4)h | 1.65 (1.58–1.71) | 22.2 (19.5–25.1)h | 0.55 (0.48–0.62) |
| Daily smokers (cigarette(s)/day) | 49.0 (47.9–50.1) | reference | 48.6 (47.0–50.2) | reference |
| 1–5 | 72.8 (71.1–74.5) | 45.3 (43.0–47.7) | ||
| 6–10 | 49.0 (47.0–50.9) | 52.7 (49.8–55.6) | ||
| 11–20 | 31.3 (29.5–33.2) | 49.5 (45.9–53.1) | ||
| 21–40 | 23.3 (19.9–27.1) | 51.5 (42.6–60.3) | ||
| >40 | 32.1 (24.0–41.5) | 53.2 (36.6–69.1) | ||
CI = confidence interval.
aIncluded 31 Pregnancy Risk Assessment Monitoring System states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011.
bPrenatal smoking cessation was defined as smoking in the 3 months before pregnancy, but not smoking during the last 3 months of pregnancy (ie, quitting).
cPostpartum relapse was defined as smoking at the time of the postpartum survey among women who had quit smoking by the last 3 months of pregnancy.
dProportion (95% CIs) among all smokers in 3 months before pregnancy (unweighted n = 29 752).
eAPRs (95% CI) among all smokers in 3 months before pregnancy; logistic regression model adjusted for maternal age, race/ethnicity, education level, marital status, pregnancy intention, parity, pre-pregnancy insurance coverage, pre-pregnancy alcohol use, site, and year of birth (unweighted n = 27 360).
fAmong all quitters by the last 3 months of pregnancy (unweighted n = 14 340).
gAPRs (95% CI) among all quitters by the last 3 months of pregnancy; logistic regression model adjusted for maternal age, race/ethnicity, education level, marital status, pregnancy intention, parity, pre-pregnancy alcohol use, site, and year of birth (unweighted n = 13 577).
hStatistically significant chi-square test (p < .001) of difference between nondaily smokers compared with all daily smokers.
Proportion of Women Who Reported Prenatal Smoking Cessation and Postpartum Relapse and Adjusted Prevalence Ratios (APRs)a
| Smoking status in 3 months before pregnancy . | Proportion of prenatal smoking cessationb . | Proportion of postpartum smoking relapsec . | ||
|---|---|---|---|---|
| % (95% CI)d . | APRe . | % (95% CI)f . | APRg . | |
| Nondaily smokers (<1 cigarette/day) | 89.7 (87.7–94.4)h | 1.65 (1.58–1.71) | 22.2 (19.5–25.1)h | 0.55 (0.48–0.62) |
| Daily smokers (cigarette(s)/day) | 49.0 (47.9–50.1) | reference | 48.6 (47.0–50.2) | reference |
| 1–5 | 72.8 (71.1–74.5) | 45.3 (43.0–47.7) | ||
| 6–10 | 49.0 (47.0–50.9) | 52.7 (49.8–55.6) | ||
| 11–20 | 31.3 (29.5–33.2) | 49.5 (45.9–53.1) | ||
| 21–40 | 23.3 (19.9–27.1) | 51.5 (42.6–60.3) | ||
| >40 | 32.1 (24.0–41.5) | 53.2 (36.6–69.1) | ||
| Smoking status in 3 months before pregnancy . | Proportion of prenatal smoking cessationb . | Proportion of postpartum smoking relapsec . | ||
|---|---|---|---|---|
| % (95% CI)d . | APRe . | % (95% CI)f . | APRg . | |
| Nondaily smokers (<1 cigarette/day) | 89.7 (87.7–94.4)h | 1.65 (1.58–1.71) | 22.2 (19.5–25.1)h | 0.55 (0.48–0.62) |
| Daily smokers (cigarette(s)/day) | 49.0 (47.9–50.1) | reference | 48.6 (47.0–50.2) | reference |
| 1–5 | 72.8 (71.1–74.5) | 45.3 (43.0–47.7) | ||
| 6–10 | 49.0 (47.0–50.9) | 52.7 (49.8–55.6) | ||
| 11–20 | 31.3 (29.5–33.2) | 49.5 (45.9–53.1) | ||
| 21–40 | 23.3 (19.9–27.1) | 51.5 (42.6–60.3) | ||
| >40 | 32.1 (24.0–41.5) | 53.2 (36.6–69.1) | ||
CI = confidence interval.
aIncluded 31 Pregnancy Risk Assessment Monitoring System states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011.
bPrenatal smoking cessation was defined as smoking in the 3 months before pregnancy, but not smoking during the last 3 months of pregnancy (ie, quitting).
cPostpartum relapse was defined as smoking at the time of the postpartum survey among women who had quit smoking by the last 3 months of pregnancy.
dProportion (95% CIs) among all smokers in 3 months before pregnancy (unweighted n = 29 752).
eAPRs (95% CI) among all smokers in 3 months before pregnancy; logistic regression model adjusted for maternal age, race/ethnicity, education level, marital status, pregnancy intention, parity, pre-pregnancy insurance coverage, pre-pregnancy alcohol use, site, and year of birth (unweighted n = 27 360).
fAmong all quitters by the last 3 months of pregnancy (unweighted n = 14 340).
gAPRs (95% CI) among all quitters by the last 3 months of pregnancy; logistic regression model adjusted for maternal age, race/ethnicity, education level, marital status, pregnancy intention, parity, pre-pregnancy alcohol use, site, and year of birth (unweighted n = 13 577).
hStatistically significant chi-square test (p < .001) of difference between nondaily smokers compared with all daily smokers.
Among women who quit smoking during pregnancy, the proportion who reported postpartum smoking relapse at the time of the survey, average 4 months postpartum, was significantly lower in nondaily smokers than all daily smokers (22.2% and 48.6%, respectively; p < .001) shown in Table 2. Among daily smokers who quit by the third month of pregnancy, the proportion who relapsed postpartum was similar regardless of daily level of cigarette consumption before pregnancy, with about half relapsing by the time of the survey. In adjusted analyses, nondaily smokers who quit were about half as likely to relapse postpartum compared to daily smokers who quit (APR = 0.55; 95% CI: 0.48–0.62). Nondaily smokers completed the survey on average 115 days after delivery compared to daily smokers who completed the survey on average of 120 days after delivery. However, this 5-day difference did not have an impact on regression estimates.
Tables 3 describes the proportion of women who reported receiving provider education about the effects of tobacco use on the infant during prenatal care, which was significantly lower for nondaily smokers (71.1%) than daily smokers (86.3%; p < .001); the highest proportion receiving education were women who smoked >40 cigarettes per day (94.2%).
Proportion of Women Who Reported Receiving Provider Education During Prenatal Carea
| Smoking status in 3 months before pregnancy . | Provider education during prenatal careb . |
|---|---|
| % (95% CI)c . | |
| Nondaily smokers (<1 cigarette/day) | 71.1 (68.2–73.8)d |
| Daily smokers (cigarette(s)/day) | 86.3 (85.5–87.1) |
| 1–5 | 82.2 (80.6–83.7) |
| 6–10 | 86.5 (85.1–87.9) |
| 11–20 | 88.9 (87.4–90.2) |
| 21–40 | 90.7 (87.8–93.0) |
| >40 | 94.2 (89.4–96.9) |
| Smoking status in 3 months before pregnancy . | Provider education during prenatal careb . |
|---|---|
| % (95% CI)c . | |
| Nondaily smokers (<1 cigarette/day) | 71.1 (68.2–73.8)d |
| Daily smokers (cigarette(s)/day) | 86.3 (85.5–87.1) |
| 1–5 | 82.2 (80.6–83.7) |
| 6–10 | 86.5 (85.1–87.9) |
| 11–20 | 88.9 (87.4–90.2) |
| 21–40 | 90.7 (87.8–93.0) |
| >40 | 94.2 (89.4–96.9) |
CI = confidence interval.
aIncluded 31 Pregnancy Risk Assessment Monitoring System (PRAMS) states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011.
bPRAMS questionnaires asked: “During any of your prenatal care visits, did a doctor, nurse, or other health care worker talk with you about any of the things listed below? Please count only discussions, not reading materials or videos.” The option of interest read, “How smoking during pregnancy could affect my baby.”
cProportion (95% CIs) among all smokers before pregnancy who answered question on PRAMS (unweighted n = 29 185).
dStatistically significant chi-square test (p < .001) of difference between nondaily smokers compared with all daily smokers.
Proportion of Women Who Reported Receiving Provider Education During Prenatal Carea
| Smoking status in 3 months before pregnancy . | Provider education during prenatal careb . |
|---|---|
| % (95% CI)c . | |
| Nondaily smokers (<1 cigarette/day) | 71.1 (68.2–73.8)d |
| Daily smokers (cigarette(s)/day) | 86.3 (85.5–87.1) |
| 1–5 | 82.2 (80.6–83.7) |
| 6–10 | 86.5 (85.1–87.9) |
| 11–20 | 88.9 (87.4–90.2) |
| 21–40 | 90.7 (87.8–93.0) |
| >40 | 94.2 (89.4–96.9) |
| Smoking status in 3 months before pregnancy . | Provider education during prenatal careb . |
|---|---|
| % (95% CI)c . | |
| Nondaily smokers (<1 cigarette/day) | 71.1 (68.2–73.8)d |
| Daily smokers (cigarette(s)/day) | 86.3 (85.5–87.1) |
| 1–5 | 82.2 (80.6–83.7) |
| 6–10 | 86.5 (85.1–87.9) |
| 11–20 | 88.9 (87.4–90.2) |
| 21–40 | 90.7 (87.8–93.0) |
| >40 | 94.2 (89.4–96.9) |
CI = confidence interval.
aIncluded 31 Pregnancy Risk Assessment Monitoring System (PRAMS) states (AK, AR, CO, DE, GA, HI, IL, MA, MD, ME, MI, MN, MO, MS, NE, NJ, NM, NY, OH, OK, OR, PA, RI, TN, TX, UT, VT, WA, WI, WV, and WY) and New York City during 2009–2011.
bPRAMS questionnaires asked: “During any of your prenatal care visits, did a doctor, nurse, or other health care worker talk with you about any of the things listed below? Please count only discussions, not reading materials or videos.” The option of interest read, “How smoking during pregnancy could affect my baby.”
cProportion (95% CIs) among all smokers before pregnancy who answered question on PRAMS (unweighted n = 29 185).
dStatistically significant chi-square test (p < .001) of difference between nondaily smokers compared with all daily smokers.
Discussion
During 2009–2011, 3% of all women and 11% of smokers in our sample reported being nondaily smokers (<1 cigarette/day) in the 3 months before pregnancy. Nondaily smokers were more similar to nonsmokers than to daily smokers with respect to demographic and pregnancy-related characteristics. Significantly higher proportions of nondaily smokers than daily smokers were in older age categories, were married, were in higher income categories, had intended pregnancies, and had private insurance, which was consistent with other studies in the general US population of nondaily smokers.2,10 Overall, nondaily smokers have lower proportions of typical high-risk factors for adverse pregnancy outcomes than daily smokers; however, this finding did not hold true for alcohol consumption.
A very high proportion of pre-pregnancy nondaily smokers (9 out of 10 women) reported that they were no longer smoking by the last 3 months of pregnancy and were significantly more likely to have quit than daily smokers, even after adjusting for potential confounders. Other studies of the general US population have found nondaily smokers report more lifetime quit attempts, are more likely to want to quit for good, and are more likely to have quit in the last year.2,10 Nondaily smokers may also be less addicted to cigarettes; although the literature on whether nondaily smokers exhibit nicotine dependence is mixed.19 This discrepancy may be largely due to the heterogeneity of smoking behaviors among those labeled as nondaily smokers.1,19 For example, in one study, nondaily smokers reported smoking between 3 to 5 cigarettes on the days smoked and smoke on average 12–14 days in the past 30 days.4 In our study, PRAMS may be capturing lighter nondaily smokers, and lighter smokers are more likely to quit smoking during pregnancy.20 For women who are ready to quit, pregnancy is a substantial motivating factor, with heightened awareness of adverse effects of smoking during pregnancy.21
In the current study, nondaily smokers as compared with daily smokers were less likely to report receiving provider education during prenatal care about how smoking can affect their baby. Tobacco screening and brief counseling in clinical settings has been rated as among the most effective and efficacious preventative health actions.22 According to the American College of Obstetricians and Gynecologists, providers should screen for any amount of tobacco use, even small amounts.22 Almost 30% of nondaily smokers reported not receiving provider education compared with only 6% of the heaviest daily smokers. Reasons for the disparity in provider education may be twofold. First, providers may not be screening for nondaily smoking. Second, nondaily smokers may be less likely to identify themselves as “smokers” to their prenatal care providers, making it challenging to identify this group.1,23,24
Although report of provider education is low, our findings that pre-pregnancy nondaily smokers have high proportions of cessation during pregnancy and low proportions of postpartum relapse suggest that they may be receptive to tobacco prevention messages that encourage life-long tobacco abstinence to reduce immediate and long-term health risks. With the heterogeneity of smoking practices among nondaily smokers, differences in the motivation to quit may partially be due to differences in smoking history and perceived susceptibility to future disease, making it potentially difficult to treat this group.25 Inquiring about a woman’s past and current behaviors may help tailor smoking cessation messages to fit each woman. For example, some nondaily smoking women may be in a stable nondaily smoking pattern or recently reduced from daily smoking, where motivating quit attempts with interventions, like the 5 As, may be effective.2,22 Whereas for other nondaily smoking women, alcohol use in social situations may be a trigger for smoking and can circumvent cessation efforts.
A noteworthy finding of our study is nondaily smokers had higher prevalence of any alcohol use (87%) and binge drinking (57%) before pregnancy than both daily and nonsmokers; however, the proportion among daily smokers was also high. This suggests smoking is an indicator for high-risk drinking, as seen by the disproportionate amount of alcohol use among smokers, making these women a target group for intervention. Compared to a recent nationally representative sample of women of reproductive age, the estimated percentages of any alcohol use and binge drinking among nonpregnant women and pregnant women were similar to the proportions found in our study among the nonsmokers.26 However, nondaily smokers in our study had almost twofold higher alcohol use and threefold higher binge drinking proportions before pregnancy than those overall national averages for nonpregnant women. This high prevalence of alcohol use among all smokers prior to and during pregnancy is of great concern given the fetal toxicity of alcohol. Alcohol use during pregnancy is associated with miscarriage, stillbirth, and a range of lifelong physical, behavioral, and intellectual disabilities.27,28 Although most nondaily smokers quit smoking by the end of pregnancy, a higher proportion used alcohol in the last 3 months of pregnancy (13%) than nonsmokers or daily smokers (both ≤7%). Provider should screen all women of reproductive age about their alcohol use as well.12 In particular, nondaily smoking could be an indicator that alerts providers of the potential for higher alcohol use and need for counseling. There is no known safe level of alcohol consumption during pregnancy, and American College of Obstetricians and Gynecologists recommends that providers give clear advice to avoid alcohol use.28
To our knowledge, this is the first population-based study to explore the characteristics of nondaily smokers and their smoking patterns during and after pregnancy. Nonetheless, this study has certain limitations. First, the PRAMS survey is based on self-report of data. Women returned the completed survey between 2 to 9 months postpartum; with variance in recall times, there may be some misclassification due to self-report of smoking patterns and other lifestyle behaviors. In addition, we classified nondaily smokers as women who reported smoking <1 cigarette per day on average. With the heterogeneity in smoking patterns among nondaily smokers, this response option may not capture all nondaily smoking women. Therefore, our estimates may underestimate the true prevalence of nondaily smoking. Previous research has shown that pregnant women have high rates of nondisclosure of smoking status, and PRAMS smoking status is not biochemically validated.29 However, PRAMS is a confidential survey, which has been shown to identify more smokers than other self-reported data sources.15 Secondly, alcohol use is also self-reported. Nondisclosure rates for alcohol use during pregnancy are not well studied, but it is likely that our rates of alcohol use before and during pregnancy are underreported given the stigma associated with the behavior. Third, we were not able to assess directly the receipt of tobacco cessation services, and only ascertained if a woman reported receiving provider education about smoking. Lastly, these results may not be generalizable to women whose pregnancies did not result in a live birth or women who delivered a live birth outside of the sites included in the study.
Nondaily smokers represented about one in ten women who smoked before pregnancy, and had relatively high rates of cessation and low rates of postpartum relapse. Although all women should be screened for and advised to avoid alcohol, the high prevalence of concurrent alcohol use among smokers surrounding pregnancy is of concern. Like daily smoking, nondaily smoking could be an indicator for this high-risk behavior. Prenatal care providers should screen for all levels of tobacco use, including nondaily and daily smoking, and should continue to educate women about the importance of smoking cessation and remaining tobacco free postpartum and beyond.
Funding
None declared.
Declaration of Interests
None declared.
Acknowledgments
This work was presented as a poster presentation at the 2015 SRNT 21st Annual Meeting—Society for Research on Nicotine and Tobacco; Philadelphia, PA; February 25–28, 2015. Pregnancy Risk Assessment Monitoring System Working Group: Alaska—Kathy Perham-Hester, MS, MPH; Arkansas—Mary McGehee, PhD; Colorado—Alyson Shupe, PhD; Delaware—George Yocher, MS; Georgia—Chinelo Ogbuanu, MD, MPH, PhD; Hawaii—Emily Roberson, MPH; Illinois—Theresa Sandidge, MA; Maine—Tom Patenaude, MPH; Maryland—Diana Cheng, MD; Massachusetts—Emily Lu, MPH; Michigan—Cristin Larder, MS; Minnesota—Judy Punyko, PhD, MPH; Mississippi—Brenda Hughes, MPPA; Missouri—Venkata Garikapaty, MSc, MS, PhD, MPH; Nebraska—Brenda Coufal; New Jersey—Lakota Kruse, MD; New Mexico—Eirian Coronado, MA; New York State—Anne Radigan-Garcia; New York City—Candace Mulready-Ward, MPH; Ohio—Connie Geidenberger PhD; Oklahoma—Alicia Lincoln, MSW, MSPH; Oregon—Kenneth Rosenberg, MD, MPH; Pennsylvania—Tony Norwood; Rhode Island—Sam Viner-Brown, PhD; Tennessee—David Law, PhD; Texas—Rochelle Kingsley, MPH; Utah—Lynsey Gammon MPH; Vermont—Peggy Brozicevic; Washington—Linda Lohdefinck; West Virginia—Melissa Baker, MA; Wisconsin—Katherine Kvale, PhD; Wyoming—Amy Spieker, MPH; CDC PRAMS Team, Applied Sciences Branch, Division of Reproductive Health. This research was supported in part by an appointment to the Research Participation Program at the CDC administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
Author notes
Corresponding Author: Van T. Tong, MPH, Centers for Disease Control and Prevention-Chamblee Campus, 4770 Buford Hwy, NE, MS F-74, Atlanta, GA 30341-3717, USA. Telephone: 770-488-6309; Fax: 770-488-6391; E-mail: vct2@cdc.gov




Comments