-
PDF
- Split View
-
Views
-
Cite
Cite
Peter P. Vitaliano, Ozge Ustundag, Soo Borson, Objective and Subjective Cognitive Problems among Caregivers and Matched Non-caregivers, The Gerontologist, Volume 57, Issue 4, August 2017, Pages 637–647, https://doi.org/10.1093/geront/gnv690
Close - Share Icon Share
Abstract
Caregivers (CGs) have been shown to do more poorly than non-caregivers (NCGs) on objective cognitive tests (Trails B and Digit Symbol Test, DST), but less is known about whether these groups differ in: (a) reports of subjective cognitive problems (SCPs, memory complaints, etc.) and (b) relationships of SCPs with objective cognitive tests, depression, and stress exposure. Such relationships are important because researchers/clinicians use SCPs as proxies for objective cognitive tests.
One hundred and twenty-two spouse CGs of persons with Alzheimer’s disease and 117 demographically matched NCG spouses were compared on Trails B and DST at baseline (T1), 1 year later (T2), and 2 years later (T3) and on SCPs at T1.
Trails B was slower in CGs than NCGs and DST declined in CGs relative to NCGs. CGs reported more SCPs than NCGs. Depression mediated group differences in Trails and DST and was also associated with SCPs. Trails B and DST explained variance in SCPs in NCGs, but not in CGs. Hours of care explained variance in SCPs in CGs, but not in NCGs.
When using SCPs to make inferences about CG cognitive function, researchers/clinicians should consider the possible influence of stress exposures and depression. The lack of associations of objective and subjective cognitive measures may be a reflection of poorer self-monitoring among CGs, a potential new area of CG research.
Spouse caregivers (CGs) of persons with Alzheimer’s disease (AD) are exposed to long-term psychological and physical demands in response to the progressive decline of their care recipients. Such experiences are often followed by greater psychosocial distress (Pinquart & Sörensen, 2003) and physiological disregulation (Vitaliano, Zhang, & Scanlan, 2003) in CGs relative to demographically similar non-caregivers (NCGs). Although CG outcomes have been examined for many decades, it was not until 2003 that CGs were shown to have poorer objective cognitive function (processing speed) than demographically matched NCGs (Caswell et al., 2003).
Since then this literature has grown to include measures of attention, memory, learning, executive function, vocabulary, and global mental status. CGs experience greater difficulty than NCGs in Attention tasks that involve switching from one learning task to a second task, especially timed tasks (Mackenzie, Smith, Hasher, Leach, & Behl, 2007; Halpert, 2010). Relative to NCGs, CGs have deficits in Memory tasks. These include free recall after brief and long delays (Halpert, 2010; Mackenzie, Wiprzycka, Hasher, & Goldstein, 2009; Palma et al., 2011) and Digit Span Backwards which assesses the ability to retain/manipulate information in working memory (Lee, Kawachi, & Grodstein, 2004). Longitudinal studies have shown that even after caregiving has ceased, further deterioration occurs in spatial working memory (Mackenzie et al., 2007).
CGs also perform worse than NCGs on learning tests (Mackenzie et al., 2009) that assess episodic/working memory and verbal fluency (memory retrieval; speed of information processing) (Caswell et al., 2003; de Vugt et al., 2006; Halpert, 2010; Vitaliano, Zhang, Young, Caswell, & Scanlan, 2009); and on Executive Function (de Vugt et al., 2006), which can have adverse effects on planning, judgment, and problem solving (Kipps & Hodges, 2005). These are important for performance on activities of daily living (ADLs). Executive function is especially important because it predicts the long-term ability to perform ADLs (Bell-McGinty, Podell, Franzen, Baird, & Williams, 2002; Cahn-Weiner, Boyle, & Malloy, 2002) in older adults. Spouse CGs of dementia patients also exhibit more vocabulary decline (Vitaliano et al., 2005; Zachary & Shipley, 1986) and poorer global cognitive functioning than do matched NCGs (de Vugt et al., 2006; Herrera, George, Angel, Markides, & Torres-Gil, 2013). Overall, most of these studies controlled for age and gender, but fewer controlled for important variables such as education and income.
Importantly, poorer cognitive function may (a) interfere with a CG’s ability to manage household tasks (Vitaliano, Murphy, Young, Echeverria, & Borson, 2011), (b) predict poorer long-term functioning in CGs relative to NCGs (Vitaliano, Echeverria, Shelkey, Zhang, & Scanlan, 2007), and (c) be relevant to accelerated frailty in dementia CGs relative to non-dementia CGs (Dassel & Carr, 2014). Many dementia CGs are also old enough to experience age-associated reductions in cognition (National Alliance for Caregiving and the American Association of Retired Persons, 1997; Salthouse & Davis, 2006), suggesting that they may enter their roles with preexisting cognitive vulnerabilities.
Unfortunately, most studies of CG cognition have been cross-sectional and have not allowed the assessment of cognitive changes in CGs relative to NCGs (Vitaliano, 2010). As such, our first goal was to examine longitudinal differences in objective cognitive measures in CGs versus NCGs. To do this we examined the Digit Symbol Test (DST), a measure of processing speed (Wechsler, 1939) and the Trails B Test, a measure of executive function. The latter is associated with several complex functions, including processing speed, attention, visual scanning, and translation of intention into action (Lezak, 1995), all of which are important to ADLs. We hypothesized that CGs would show greater deficits in Trails B than NCGs; and, that depressive symptoms would explain this difference at baseline and across time. We did this because depression is a major correlate of CG status (Pinquart & Sörensen, 2003) and cognitive function (Kizilbash, Vanderploeg, & Curtiss, 2002).
In addition to assessing, CG versus NCG differences in objective cognitive function, we examined differences in subjective cognitive problems (SCPs), an understudied area among CGs. SCPs are used to acquire information about cognitive complaints in health care and research settings. SCPs may be a function of poorer objective cognitive function (Jonker, Geerlings, & Schmand, 2000; Schofield et al., 1997; Wilson & Evans, 1996) and psychological distress (Jonker et al., 2000). Hence, our second goal was to examine whether CGs reported more SCPs than NCGs and if so, whether this was related to depression. Finally, we assessed whether relationships between subjective and objective cognitive measures differed in CGs and NCGs, and if so, whether SCPs were predicted differently in each group by objective cognitive measures, depression, and an indicator of CG stress exposure (hours of care).
Depressive symptoms are common and important correlates of: (a) SCPs (Jonker et al., 2000), (b) objective cognitive measures (Bremner, 1999; Levy, Dachir, Arbel, & Kadar, 1994; Lupien et al., 1994; Newcomer, Craft, Hershey, Askins, & Bardgett, 1994), and (c) caregiving (Covinsky et al., 2003; Gallagher-Thompson et al., 2006; Wisniewski et al., 2003). Both depression and stressors influence brain function (Kim & Diamond, 2002; Roozendaal, McReynolds, & McGaugh, 2004). Depression may also be related to cognitive processes (Baune, Suslow, Arolt, & Berger, 2007) because it is associated with elevations in stress hormones (Erickson, Drevets, & Schulkin, 2003; Lee et al., 2007), strong physiological correlates of CG status (Vitaliano et al., 2003).
Methods
Design and Participants
We recruited CG couples from the general community in Washington State using printed or electronic media, physicians’ offices, the University of Washington (UW) AD registry, and the Alzheimer’s Association. Criteria for care recipient inclusion were that one had to be living with one’s spouse CG, be aged 55 years or older, and have a Diagnostic and Statistical Manual (DSM)-IV Diagnosis of dementia of the Alzheimer’s type or possible/probable primary degenerative dementia (McKhann et al., 1984; the criteria used when these data were collected in 2000). CGs had to be 55 years and older, function independently, and be the primary CG for their spouse care recipient. The diagnosis of dementia was required for AD care recipients (spouses of CGs), but not for CGs, NCGs or spouses of NCGs.
Demographically similar NCGs were recruited from senior centers, retirement organizations, and media. NCGs and their spouses had to be aged 55 years or older, functioning independently, and not providing care for anyone on a regular basis. During recruitment, we asked all prospective CGs and NCGs if they were physically mobile and able to perform ADLs (e.g., grooming, cleaning, and managing finances). These responses were then verified during initial assessment (Ware, Kosinski, & Keller, 1996). To verify clinical cognitive status, care recipients with AD (spouses of CGs) and spouses of NCGs were assessed with the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975). AD care recipients had to score at 27 or below and spouses of NCGs had to score above 27 (Table 1). The UW Institutional Review Board approved the study and informed consent was obtained. In addition to being married to someone with AD and self-reporting as their CG, we examined the number of hours of care per day CGs spent performing ADLs and caring for their spouses as an indicator of chronic stress. One week prior to each visit, we called the CGs/NCGs and reminded them to complete the home questionnaires that had been sent 2 weeks earlier. We oriented them to the measures, which included a place to record the average hours per day that they had spent caring for their spouses during that week. To verify that CGs had exposures to caregiving relative to NCGs, we also collected this information on NCGs.
CG and NCG Demographic, Health, Psychosocial, and Cognitive Measures
| Variables . | Caregivers n = 122 . | Non-caregivers n = 117 . |
|---|---|---|
| Demographic/Health Factors | ||
| %Women | 62 | 64 |
| % Caucasian/% Black | 94/6 | 92/8 |
| Age (yrs) (Ma ± SDb) | 71.7±8.9 | 70.2±7.2 |
| Education (yr) | 15.2±2.6 | 15.2±2.6 |
| Income ($)c | 52.0±31.0 | 50.7±26.5 |
| # Years married | 42.1±15.3 | 40.5±13.7 |
| %AntiHypertensiveMeds | 38 | 36d |
| % Sleep Meds | 3 | 2 |
| % Psychotropic Medse | 31 | 15** |
| % CHD | 18 | 17 |
| % Hypertension | 39 | 33 |
| % Diabetes | 8 | 6 |
| % Stroke | 3 | 3 |
| Psychosocial Measures | ||
| % Current depression | 0 | 0 |
| % History of Depression | 6 | 7 |
| Hamilton depression T1 | 2.43±3.1 | 1.10±2.8*** |
| Hamilton depression T2 | 2.51±2.8 | 0.85±2.7*** |
| Hamilton depression T3 | 2.62+3.1 | 0.86+1.8*** |
| Objective Cognitive Measures | ||
| Digit Symbol T1 (M ± SD) | 46.0±10.8 | 49.3±11.8*** |
| Digit Symbol T2 (M ± SD) | 45.7±11.6 | 49.5±11.6*** |
| Digit Symbol T3 (M ± SD) | 45.0±11.0 | 49.5±12.0*** |
| Trails B T1 (M ± SD) | 4.54 ± .36f | 4.45 ± .38*,g |
| Trails B T2 (M ± SD) | 4.55 ± .38 | 4.48 ± .38*,g |
| Trails B T3 (M ± SD) | 4.53 ± .39 | 4.49 ± .38h |
| Subjective | ||
| Cognitive Measure | 1.24±1.62 | 0.84±1.42* |
| Validity Variables | ||
| MMSE T1 (M ± SD) for spouses of CGs/NCGs | 17.0±6.6 | 28.3±1.8*** |
| MMSE T2 | 14.8±8.1 | 28.2±2.3*** |
| MMSE T3 | 13.5±8.4 | 28.5±2.2*** |
| Average hours Care/day T1 | 7.0±8.2 | 1.0±3.6*** |
| Average hours Care/day T2 | 9.0±8.6 | 1.2±3.6*** |
| Average hours Care/day T3 | 7.0±8.1 | 1.3±3.6*** |
| Variables . | Caregivers n = 122 . | Non-caregivers n = 117 . |
|---|---|---|
| Demographic/Health Factors | ||
| %Women | 62 | 64 |
| % Caucasian/% Black | 94/6 | 92/8 |
| Age (yrs) (Ma ± SDb) | 71.7±8.9 | 70.2±7.2 |
| Education (yr) | 15.2±2.6 | 15.2±2.6 |
| Income ($)c | 52.0±31.0 | 50.7±26.5 |
| # Years married | 42.1±15.3 | 40.5±13.7 |
| %AntiHypertensiveMeds | 38 | 36d |
| % Sleep Meds | 3 | 2 |
| % Psychotropic Medse | 31 | 15** |
| % CHD | 18 | 17 |
| % Hypertension | 39 | 33 |
| % Diabetes | 8 | 6 |
| % Stroke | 3 | 3 |
| Psychosocial Measures | ||
| % Current depression | 0 | 0 |
| % History of Depression | 6 | 7 |
| Hamilton depression T1 | 2.43±3.1 | 1.10±2.8*** |
| Hamilton depression T2 | 2.51±2.8 | 0.85±2.7*** |
| Hamilton depression T3 | 2.62+3.1 | 0.86+1.8*** |
| Objective Cognitive Measures | ||
| Digit Symbol T1 (M ± SD) | 46.0±10.8 | 49.3±11.8*** |
| Digit Symbol T2 (M ± SD) | 45.7±11.6 | 49.5±11.6*** |
| Digit Symbol T3 (M ± SD) | 45.0±11.0 | 49.5±12.0*** |
| Trails B T1 (M ± SD) | 4.54 ± .36f | 4.45 ± .38*,g |
| Trails B T2 (M ± SD) | 4.55 ± .38 | 4.48 ± .38*,g |
| Trails B T3 (M ± SD) | 4.53 ± .39 | 4.49 ± .38h |
| Subjective | ||
| Cognitive Measure | 1.24±1.62 | 0.84±1.42* |
| Validity Variables | ||
| MMSE T1 (M ± SD) for spouses of CGs/NCGs | 17.0±6.6 | 28.3±1.8*** |
| MMSE T2 | 14.8±8.1 | 28.2±2.3*** |
| MMSE T3 | 13.5±8.4 | 28.5±2.2*** |
| Average hours Care/day T1 | 7.0±8.2 | 1.0±3.6*** |
| Average hours Care/day T2 | 9.0±8.6 | 1.2±3.6*** |
| Average hours Care/day T3 | 7.0±8.1 | 1.3±3.6*** |
Notes:aMean.
bStandard Deviation.
c$1,000 units.
dIn women.
eantidepressants/anxiety.
fNatural log of seconds.
gSignificant with covariates for age, gender, Abstraction IQ at T1 and T3, ethnicity, gender-hormone replacement therapy.
hp = .09.
*p < .05. **p < .01. ***p < .001.
CG and NCG Demographic, Health, Psychosocial, and Cognitive Measures
| Variables . | Caregivers n = 122 . | Non-caregivers n = 117 . |
|---|---|---|
| Demographic/Health Factors | ||
| %Women | 62 | 64 |
| % Caucasian/% Black | 94/6 | 92/8 |
| Age (yrs) (Ma ± SDb) | 71.7±8.9 | 70.2±7.2 |
| Education (yr) | 15.2±2.6 | 15.2±2.6 |
| Income ($)c | 52.0±31.0 | 50.7±26.5 |
| # Years married | 42.1±15.3 | 40.5±13.7 |
| %AntiHypertensiveMeds | 38 | 36d |
| % Sleep Meds | 3 | 2 |
| % Psychotropic Medse | 31 | 15** |
| % CHD | 18 | 17 |
| % Hypertension | 39 | 33 |
| % Diabetes | 8 | 6 |
| % Stroke | 3 | 3 |
| Psychosocial Measures | ||
| % Current depression | 0 | 0 |
| % History of Depression | 6 | 7 |
| Hamilton depression T1 | 2.43±3.1 | 1.10±2.8*** |
| Hamilton depression T2 | 2.51±2.8 | 0.85±2.7*** |
| Hamilton depression T3 | 2.62+3.1 | 0.86+1.8*** |
| Objective Cognitive Measures | ||
| Digit Symbol T1 (M ± SD) | 46.0±10.8 | 49.3±11.8*** |
| Digit Symbol T2 (M ± SD) | 45.7±11.6 | 49.5±11.6*** |
| Digit Symbol T3 (M ± SD) | 45.0±11.0 | 49.5±12.0*** |
| Trails B T1 (M ± SD) | 4.54 ± .36f | 4.45 ± .38*,g |
| Trails B T2 (M ± SD) | 4.55 ± .38 | 4.48 ± .38*,g |
| Trails B T3 (M ± SD) | 4.53 ± .39 | 4.49 ± .38h |
| Subjective | ||
| Cognitive Measure | 1.24±1.62 | 0.84±1.42* |
| Validity Variables | ||
| MMSE T1 (M ± SD) for spouses of CGs/NCGs | 17.0±6.6 | 28.3±1.8*** |
| MMSE T2 | 14.8±8.1 | 28.2±2.3*** |
| MMSE T3 | 13.5±8.4 | 28.5±2.2*** |
| Average hours Care/day T1 | 7.0±8.2 | 1.0±3.6*** |
| Average hours Care/day T2 | 9.0±8.6 | 1.2±3.6*** |
| Average hours Care/day T3 | 7.0±8.1 | 1.3±3.6*** |
| Variables . | Caregivers n = 122 . | Non-caregivers n = 117 . |
|---|---|---|
| Demographic/Health Factors | ||
| %Women | 62 | 64 |
| % Caucasian/% Black | 94/6 | 92/8 |
| Age (yrs) (Ma ± SDb) | 71.7±8.9 | 70.2±7.2 |
| Education (yr) | 15.2±2.6 | 15.2±2.6 |
| Income ($)c | 52.0±31.0 | 50.7±26.5 |
| # Years married | 42.1±15.3 | 40.5±13.7 |
| %AntiHypertensiveMeds | 38 | 36d |
| % Sleep Meds | 3 | 2 |
| % Psychotropic Medse | 31 | 15** |
| % CHD | 18 | 17 |
| % Hypertension | 39 | 33 |
| % Diabetes | 8 | 6 |
| % Stroke | 3 | 3 |
| Psychosocial Measures | ||
| % Current depression | 0 | 0 |
| % History of Depression | 6 | 7 |
| Hamilton depression T1 | 2.43±3.1 | 1.10±2.8*** |
| Hamilton depression T2 | 2.51±2.8 | 0.85±2.7*** |
| Hamilton depression T3 | 2.62+3.1 | 0.86+1.8*** |
| Objective Cognitive Measures | ||
| Digit Symbol T1 (M ± SD) | 46.0±10.8 | 49.3±11.8*** |
| Digit Symbol T2 (M ± SD) | 45.7±11.6 | 49.5±11.6*** |
| Digit Symbol T3 (M ± SD) | 45.0±11.0 | 49.5±12.0*** |
| Trails B T1 (M ± SD) | 4.54 ± .36f | 4.45 ± .38*,g |
| Trails B T2 (M ± SD) | 4.55 ± .38 | 4.48 ± .38*,g |
| Trails B T3 (M ± SD) | 4.53 ± .39 | 4.49 ± .38h |
| Subjective | ||
| Cognitive Measure | 1.24±1.62 | 0.84±1.42* |
| Validity Variables | ||
| MMSE T1 (M ± SD) for spouses of CGs/NCGs | 17.0±6.6 | 28.3±1.8*** |
| MMSE T2 | 14.8±8.1 | 28.2±2.3*** |
| MMSE T3 | 13.5±8.4 | 28.5±2.2*** |
| Average hours Care/day T1 | 7.0±8.2 | 1.0±3.6*** |
| Average hours Care/day T2 | 9.0±8.6 | 1.2±3.6*** |
| Average hours Care/day T3 | 7.0±8.1 | 1.3±3.6*** |
Notes:aMean.
bStandard Deviation.
c$1,000 units.
dIn women.
eantidepressants/anxiety.
fNatural log of seconds.
gSignificant with covariates for age, gender, Abstraction IQ at T1 and T3, ethnicity, gender-hormone replacement therapy.
hp = .09.
*p < .05. **p < .01. ***p < .001.
CGs and NCGs were matched on demographic variables age, gender, race, education, and income (Table 1). We examined CGs and NCGs at study entry (Time 1 = T1), 1 year after T1 (T2), and 2 years after T1 (T3). We performed face-to-face interviews in our offices at T1 and T2. At T3, 87% of the interviews were in our offices and 13% in the participants’ homes. At T1, we sampled 130 spouse CGs and their spouses (AD care recipients) and 125 NCG spouses (and their AD-free spouses). In 2 years, 3 CGs and 1 NCG died, 4 CGs and 4 NCGs moved, 1 CG and 1 NCG reported being too ill and 2 NCGs refused to continue. This left 122 CGs and 117 NCGs. Our good 2-year retention rate may have resulted from strategies adopted after completing three longitudinal studies of this kind: We obtained next of kin/friend contacts in case communications were lost between our staff and the CGs and NCGs. This allowed us to maintain regular contact with participants via two phone calls and greeting cards during each time interval. Between study entry and Time 2, all care recipients were still living at home, but by T3, 17% of the spouses of CGs and 7% of the spouses of NCGs had entered homes (p < .05). The locations of the AD care recipient (home vs. nursing home) at the T3 interview were examined in subsequent analyses to assess their relevance to the results.
Measures
Intelligence Measures
To control for intelligence, we used the vocabulary and abstraction measures from the Shipley Institute of Living Scale (SILS) (Zachary & Shipley, 1986).
Vocabulary
This is a measure of crystallized intelligence which is derived from prior learning (Cattell, 1963, 1971). It includes 40 multiple-choice items (range is 0–40). In this study, the coefficient alpha was .87 and intraclass correlation was .73 (p < .01).
Abstraction
This is a measure of fluid intelligence (Cattell, 1963, 1971). This includes the ability to solve problems and think/reason abstractly, processes believed to be independent of learning and education. This measure includes 20 reasoning items, each with a sequence of numbers, letters, or words with their final element omitted. For each item, the respondent must complete the sequence. Total score is number correct out of 20. Alpha was .84 and the intraclass correlation was .70 (p < .01).
Cognitive Function Measures
The Trail Making Test B
(Arbuthnott & Frank, 2000; Army Individual Test Battery, 1944; Reitan, 1958) It measures executive function or the control/regulation of cognitive processes. These include working memory, reasoning, problem solving and flexibility, planning, and execution. Trails B consists of 25 circles distributed over a sheet of paper. The circles include numbers (1–13) and letters (A–L). The subject must draw lines to connect the circles in an ascending pattern, and must also alternate between the numbers and letters (i.e., 1-A-2-B-3-C, etc.); and connect the circles as quickly as possible, without lifting the pen/pencil from the paper. The subject is timed as he/she connects the “trail.” If an error is made, this is noted and he/she is allowed to correct it. Errors affect the score because the correction of errors is included in the task’s completion time. Results are reported as the number of seconds to complete the task; for example, higher scores, greater impairment.
The Digit Symbol Test (DST)
This test assesses memory, processing speed, complex attention, psychomotor speed, cognitive-motor translation, and concentration (Lezak, 1995; Wechsler, 1939). A paired array of 10 digits (0–9) and abstract symbols is shown. Subjects must write in the correct corresponding digit from the first array next to another array of symbols. Test–retest reliability is 0.89 (Dikmen, Heaton, Grant, & Temkin, 1999). The score is # correct in 90s.
Subjective Cognitive Problems (SCPs)
It includes eight items in which the respondent is asked to report whether he/she has difficulties (“yes-no”) in concentration, attention, forgetting, disorientation, not completing things, reacting slowly, confusion, and making mistakes. These items were chosen by comparing the item content of scales with 1 item (Benito-León, Mitchell, Vega, & Bermejo-Pareja, 2010; Schofield et al., 1997), 4 items (Jorm et al., 1997), 10 items (thinking more slowly, confusion, concentration) (Schmand, Jonker, Geerlings, & Lindeboom, 1997), and 24 items (Snitz et al., 2012) Given the response burden in this study, we only included eight items. These items were used in the most comprehensive measure of SCPs (Snitz et al., 2012). The eight items had a coefficient alpha of .79.
Psychosocial, Health, and Health Habit Measures
The Hamilton Depression Rating Scale (Ham-D)
(Hamilton, 1960) [24 items] This scale assesses depressive mood symptoms present for at least 2 days, from 0 (absent) to 4 (severe) (e.g., sadness, guilt, sleep problems, etc.). The mean alpha for Ham-D for T1-T3 was .85.
Clinical Depression
This measure was assessed by the Structured Clinical Interview for the DSM-IV (Spitzer, Williams, Gibbon, & First, 1992). The same trained interviewer performed all face-to-face ratings. This was done for current and past depression (previous 5 years).
Physical Illnesses and Medications
These were assessed using self-report and medical records (International Classification of Diseases, 10th revision) for diagnostic tests and dates. These were coded for the previous 5 years. Eighteen percent of the medical records did not include an illness, and 22% of records did not list a medication, but self-reports did. Self-reports were used for participants (6%) who did not have medical records.
Hormone Replacement Therapy
This therapy was asked for the past year.
Inferential Analyses
Repeated measures, analyses of covariance, and regression analyses examined relationships between CG status and objective and subjective cognitive function. Regressions were also performed to examine mediation and whether relationships of objective and subjective cognition function differed in CGs versus NCGs. Analyses controlled for variables known to be related to cognitive function/decline (see later).
Results
Sample Description
The groups did not differ in demographics, years married, illnesses, and medication use (Table 1). CGs had higher Hamilton scores than NCGs at T1, t(235.6) = – 3.49, p = .001; T2, t(236.9) = – 4.62, p = .001; and T3, t(195.1) = −5.44, p = .001. No CGs or NCGs had current clinical depression, but 6% of CGs and 7% of NCGs had a history of clinical depression. Using cutoffs for the 24 item Hamilton, we observed that over two years: 77%–79.5% of the CGs were not depressed, 13.9%–15.5% were mildly depressed, 3.2%–5.7% were moderately depressed and 0.8%–4.1% were severely depressed. In contrast, 92.3%–94.9% of the NCGs were not depressed, 2.6%–5.2% were mildly depressed, 0.9% were moderately depressed and 0.0%–2.6% were severely depressed. CGs were also higher on obesity and psychotropic medicine use. As expected from our design, at Time 1 care recipient spouses of CGs had lower MMSE scores than spouses of NCGs, and spouse CGs spent more hours per day providing care for their spouses (M = 7.0; SD = 8.2) than did spouses of NCGs (M = 1.0; SD = 3.6). At study entry CGs had been caring for their spouses for a median of 3.68 years.
Relationships of Caregiver Status with Objective Cognitive Function
Digit Symbol
DST was examined because we were interested in its relationship with subjective reports of cognitive difficulties. However, we do not include analyses of caregiver status with DST at Times 1–3 as these data were reported previously (Vitaliano et al., 2009). In this work, we showed that despite being lower on DST at study entry (3.30 points or 0.3 SD), over the next 2 years CGs still declined further relative to NCGs, who did not decline (mean for CGs was 45.0 vs. 49.5 for NCGs, effect size = .38 (Table 1).
Trails B
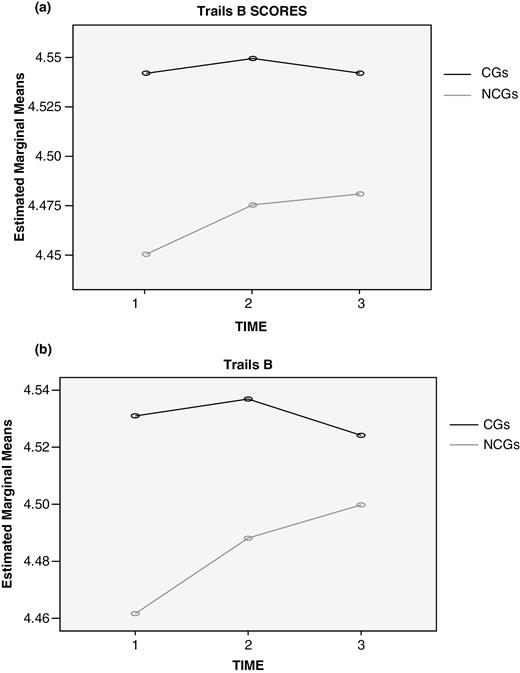
Table 1 includes means and SDs for CGs and NCGs at each of the three times. They are presented in natural logs because Trails B is measured in seconds to completion, and its distribution was skewed; for example, a mean of 4.54 corresponds to a raw mean of about 94s a repeated measures analysis was conducted for ln Trails B with: (a) the three time points as the within factor, (b) CG status as the between factor, and (c) 10 covariates (age, education, Shipley Vocabulary at Times 1 and 3, Shipley Abstraction at Times 1 and 3, race, income, and dummy variables for gender and hormone replacement therapy [HRT]). No correction for lack of compound symmetry was necessary as the Mauchly’s test of sphericity was nonsignificant, W (2) = .99, ns, approximate χ2 (2) = 2.1. There was no interaction between the time factor and groups, F(2, 444) = 0.55. We also performed this test with psychotropic medications in the model and obtained similar results, F(2, 442) = 0.3.
Given the lack of a group by time interaction, time was collapsed and an analysis of covariance (ANCOVA) was performed with Mean Trails B as the dependent variable, CG status as the between factor and the covariates used in the repeated measures analysis. CGs took longer than NCGs on Average Trails B, F(1, 222) = 3.95, p = .048, eta square = .02. Interestingly, this result occurred because of Times 1 and 2 and not Time 3, as the difference was nonsignificant at T3. This appeared to be because NCGs increased over time, but CGs did not.
We then attempted to understand why CGs took longer on Trails B than NCGs by assessing whether higher depression in CGs (Table 1) could help explain slower scores in CGs. We performed a regression with Average Trails B as the outcome measure. We entered the same ten covariates as in the ANCOVA, then CG status, which was significant, t(1, 222) = −1.98, p = .048, β = .10, overall F(11, 222) = 17.00, p = .000, adjusted R2 = .43. Average mean Hamilton score (T1 − T3) was then entered and CG status was no longer significant in the presence of depression (β dropped from −.10 to − .03), t(1, 221) = −0.63, ns, but depression was significant, t(1, 221) = 3.57, p = .000, β = .19, F(12, 221) = 17.46, p = .000, adjusted R2 = .03. The analysis was then repeated with psychotropic medications and this term was not significant, t(1, 220) = −0.63, ns. Figure 1a shows plots for CG and NCG differences in Trails B. Figure 1b shows plots when depression was controlled.
(a) Means for CGs (in black) and NCGs (in grey) on Trails B at Times 1–3. (b) Means for CGs (in black) and NCGs (in grey) on Trails B at Times 1–3 after controlling for mean depression over Times 1–3.
Relationships of Caregiver Status with Subjective Cognitive Problems
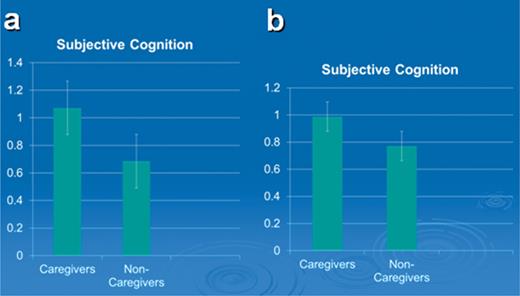
We compared SCPs in CGs versus NCGs at Time 1 by performing an ANCOVA controlling for eight of the 10 covariates as in the ANCOVA for Trails B (Time 3 scores for Shipley Vocabulary and Abstraction were excluded because SCP was only assessed at Time 1). CGs were higher on SCPs than were NCGs, F(1, 224) = 6.61, p = .01, eta square = .03 (Table 1).
A regression was then performed to assess the influence of depression and psychotropic medications on SCPs. In this model the eight covariates in the ANCOVA above were entered, then depression, and finally, psychotropic medications. The model with the eight covariates was F(8, 225) = 0.95, ns. When CG status was added to the model, it was significant, t(1, 224) = −2.28, p = .02, β = −.15. When Hamilton Depression and psychotropic medications entered the equation, depression was still significant, t(1, 222) = 3.39, p = .001, β = .24, as were psychotropic medications, t(1, 222) = 2.27, p = .02, β = .16; however, CG status was not significant, t(1, 222) = −0.99 and the β for CG status dropped from –.15 to −.065, ns. Indeed, when introduced alone, depression or psychotropic medications would have reduced the relationship of CG status with SCPs. Figure 2a and b shows the relative differences in SCP means for CGs and NCGs before and after depression was controlled.
(a) CGs report more subjective cognitive problems than NCGs. (b) CGs do not differ from NCGs when Hamilton depression at T1 is controlled.
Differences in CGs and NCGs in Relationships of SCPs with Trails B
Given relationships of CG status with SCPs and the finding that higher SCPs in CGs versus NCGs were influenced by depression, we examined whether SCPs were related differently to an objective measure of cognitive function (Trails B) in CGs and NCGs and if so what might account for such differences? To address this question, a model was examined with SCP as the outcome variable, the eight covariates (step 1), Hamilton depression at Time 1 and psychotropic medications (step 2), CG status and Trails B at Time 1 (step 3), and the interaction of CG status and Trails B (step 4). In step 4, the model was not significant, F (8, 224) = 1.04. In step 2, Hamilton depression, t(1, 222) = 3.61, p = .000 and psychotropic medications were added, t(1,222) = 2.36, p = .02, F (10, 222) = 3.98, p = .000, adjusted R2 = .11. In step 3, Trails B at Time 1 and CG status entered, and as in the previous model, CG status was not significant in the presence of depression and psychotropic medications, t(1, 220) = −0.89 and Trails B showed a trend, t(1, 220) = 1.82, p = .07, F(12, 220) = 3.73, adjusted R2 = .12. In step 4, the interaction of CG status and Trails B was entered and it was significant, t(1, 219) = 1.99, p = .048, F(13, 219) = 3.79, adjusted R2 = .14.
To interpret this interaction, we performed two separate regressions on SCPs for CGs and NCGs using the same covariates and two other variables, hours of care and Trails B at Time 1. In the CG analysis, 10 covariates were included as in the above two group analysis, F(10, 106) = 2.35, p = .015, adjusted R2 = .10. These included the eight covariates used earlier, psychotropic medications, and Hamilton depression at Time 1. In this model, depression was significant, t(1, 106) = 2.58, p = .01, β = .26 and psychotropic medications showed a trend, t(1, 106) = 1.71, p = .09. In the next step, hours of care explained additional variance in SCPs, t(1, 105) = 2.38, p = .019, β = .22, but Trails B was not significant, either independently, t(1, 105) = - .059 or in the model with hours of care. The overall F(11, 105) was 2.74, p = .004, adjusted R2 = .14, R2 change = .04.
For NCGs, the same ten covariates yielded an F(10, 105) = 1.78, p = .07, adjusted R2 = .06. In this model, depression was significant, t(1, 105) = 2.24, p = .027, β = .24, but psychotropic medications were not, t(1, 105) = 1.17, ns. After adding Trails B and hours of care to the model, Trails B was significant, t(1, 104) = 3.82, p = .000, β = .41 and it increased the adjusted R2 to .17, almost tripling the variance explained by the previous model, F(11, 104) = 3.15, p = .001. This suggested a relationship between an objective cognitive measure (Trails B) and subjective cognitive difficulties in NCGs that did not occur in CGs. Hours of care was not significant when entering the model without Trails B, t(1, 104) = 1.7 or in the presence of Trails B, t(1, 104) = 1.56.
Differences in CGs and NCGs in Relationships of SCPs with DST
As in the models for Trails B, a model for DST was examined with SCP as the outcome variable, the eight covariates (step 1), Hamilton depression at Time 1 and psychotropic medications (step 2), CG status and DST at Time 1 (step 3), and the interaction of CG status and DST (step 4). In step 1, the model was significant, F(8, 222) = 1.98, p = .05, adjusted R2 = .03. In step 2, depression, t(1, 220) = 3.17, p = .002 and psychotropic medications were added, t 1, 220) = 2.22, p = .028, F(10, 220) = 4.34, p = .000, adjusted R2 = .13. In step 3, DST at Time 1 and CG status entered. CG status was not significant, t(1, 218) = −0.36, but DST was significant, t(1, 218) = −2.08, p = .038, F(12, 218) = 4.05, adjusted R2 = .14. In Step 4, the interaction of CG status and DST was entered and it was significant, t(1, 217) = 1.99, p = .048, F(13, 217) = 4.12, adjusted R2 = .15.
To interpret this interaction, we performed two separate regressions on SCPs for CGs and NCGs using the same covariates and two other variables, hours of care at Time 1and DST at Time 1. In the CG analysis, 10 covariates were included as in the above two group analysis, F(10, 108) = 2.58, p = .008, adjusted R2 = .12. These included the eight covariates used earlier, psychotropic medications and depression at Time 1. In this model, depression was significant, t(1, 108) = 2.31, p = .02, β = .24, but psychotropic medications were not, t(1, 108) = 1.34, ns. In the next step, we observed that hours of care explained additional variance in SCPs, t(1, 107) = 2.70, p = .008, β = .25, but Trails B was not significant either independently, t(1, 107) = - .63 or in the model with hours of care. The overall F(11, 107) was 2.35, p = .01, adjusted R2 = .16, R2 change = 04.
For NCGs, the 10 covariates yielded an F(10, 101) = 2.36, p = .015, adjusted R2 = .11. In this model, depression was significant, t(1, 101) = 2.60, p = .01, β = .29, but psychotropic medications were not, t(1, 101) = 1.24, ns. After adding DST and hours of care to the model, DST was significant, t(1, 99) = −3.11, p = .002, β = −.31 and it increased the adjusted R2 to .21, almost doubling the variance explained by the model, F(11, 99) = 3.28, p = .001. This suggested a relationship between DST and subjective cognitive difficulties in NCGs that did not occur in CGs. In contrast, hours of care was not significant when entering the model without DST, t(1, 99) = 1.8 or in the presence of DST, t(1, 98) = 1.41.
Discussion
Given the importance of spouse CGs of persons with AD and their potential risk for poorer cognitive function and greater cognitive complaints relative to spouse NCGs, we assessed whether: “spouse CGs had poorer scores on objective cognitive measures (Trails B, DST) and greater SCPs than spouse NCGs? If so, whether differences in depression in CGs versus NCGs explain these relationships? Finally, were relationships of SCPs with Trails B and DST different in CGs and NCGs and if so, did different factors influence these relationships in each group?”
We attempted to shed light on these questions because a growing literature has shown that CGs have poorer objective cognitive function than NCGs. However, a few of these studies have been longitudinal (Mackenzie et al., 2007; Vitaliano et al., 2005), with one study showing a higher risk of developing dementia in spouses of persons with AD than spouses of persons without AD (Norton et al., 2010). Here we assessed longitudinal relationships of CG status with Trails B, a measure of executive function that is predictive of difficulties in ADLs (Grigsby, Kaye, Baxter, Shetterly, & Hamman, 1998). CGs had previously been shown to perform more poorly than NCGs on Trails B (de Vugt et al., 2006) in cross-sectional analysis. Our work supports these findings and also shows that differences in CGs and NCGs may weaken over time because NCGs begin to exhibit slower times.
Consistent with the literature (Pinquart & Sörensen, 2003), CGs had greater depression than NCGs; and three to four times as many CGs versus NCGs (20%–23% of CGs vs. 5%–8% of NCGs) were in the mild-to-severe range of depression over 2 years (Hamilton, 1960). Moreover, depressed mood was shown to be a mediator of group differences in Trails B (as it was for DST; Vitaliano et al., 2009). This finding supports work by Kizilbash et al. (2002) who found that greater depression was associated with slower Trails B scores. Importantly, the combination of poor executive function and depression is associated with worse physical functioning (Sanders, Lyness, Eberly, King, & Caine, 2006), which may have long-term implications for CG health and their care for their spouses.
Our findings also support work which showed that persons under chronic stress report more cognitive difficulties than do persons not under chronic stress (Linden, Keijsers, Eling, & Schaijk, 2005; Ohman, Nordin, Bergdahl, Birgander, & Neely, 2007; Sandström et al., 2011); and, that this relationship was mediated by greater depression in CGs. Moreover, depression was associated with SCPs within each group, which supports previous research (Benito-León et al., 2010; Jonker et al., 2000).
In NCGs, objective cognitive function (Trails B, DST) predicted SCPs, a result supportive of previous work (Benito-León et al., 2010; Jonker et al., 2000; Schofield et al., 1997; Wilson & Evans, 1996). In contrast, in CGs, SCPs were not predicted by Trails B or DST, but by stress exposure (hours of care). Although a relationship between a measure of chronic stress and SCP was expected (Öhman, Nordin, Bergdahl, Birgander, & Neely, 2007), it is unclear why CGs who ranked high on SCPs did not rank low on objective measures. One possibility is that CGs may be poorer at self-monitoring than NCGs. This may occur because depression is associated with poor emotion-regulation in NCGs (Friedman & Miller-Herringer, 1991) and CGs (Hooley, Orley, & Teasdale, 1986; Vitaliano, Russo, Bailey, Young, & McCann, 1993a), and CGs may not be able to self-regulate as well as NCGs. Poor self-regulation in CGs has been studied via expressed emotion (EE) or the heightened criticism of one’s care recipient (Vaughn, Snyder, Jones, Freeman, & Falloon, 1984), where CGs were significantly higher in EE (37%) than NCGs (15%) (Vitaliano, Young, Russo, Romano, & Magana-Amato, 1993b). Interestingly, emotionally arousing stimuli are supposed to facilitate cognition and benefit brain structures that control declarative memory performance, but this does not seem to occur in CGs (Palma et al., 2011). This may be important to long-term CG function because cognitive processes are usually a catalyst for clinical/neuropsychological follow-up and feedback.
This study has a number of limitations. First, SCPs were only assessed at the beginning of the study and so we could not examine differential changes in SCPs with changes in objective assessments and hours of care in CGs versus NCGs. Also, our measure of SCP was much less comprehensive than that of Snitz et al. (2012) which had a larger and more inclusive item pool. This would have improved its reliability and content validity. We also relied on self-reports of hours of care and do not have data to document their reliability/validity, other than the fact that CGs reported many more hours of care at all times than did NCGs. A weekly log would have been more reliable. Finally, after several covariates and depression were controlled in the NCG analyses, DST explained 10% additional variance and Trails B explained 11% additional variance in SCPs. In contrast, in CGs, no such variance was explained. Alternatively in CGs, hours of care explained 4% additional variance in SCPs, but not in NCGs. These are small ESs, but the pattern of differences for each group and the consistency of results for two highly used cognitive measures, suggest this area should receive further study. The clinical implications of these results are unknown, so researchers/clinicians should assess whether CG self-reports can be used to assess their ability to function and care for their loved ones. Finally, although the DSM-IV criteria were used to diagnose care recipients with AD Dementia, we believe they would also have received this diagnosis using the DSM-V. This is because the: (a) DSM-IV diagnoses were made by referring diagnosticians, (b) care recipients’ mean MMSE score at entry was 17 and consistent with late-mild or early-moderate stage dementia, and (c) decline to a mean score of 13.5 over 2 years was consistent with the expected AD trajectory. Moreover, the CG time reported by their spouses of 7hr/day at baseline is consistent with individuals at this level of dementia.
We believe that this study has several merits. (a) To our knowledge, this is the first study to examine relationships of objective cognitive measures and cognitive complaints in CGs versus NCGs and our exposure versus no exposure longitudinal design allowed us to control for aging’s effects on cognition over time (Jorm, Korten, & Henderson, 1987; Park, 1996) (Figure 1). (b) Few studies have examined longitudinal relationships of CG status with objective cognitive functioning and potential mediators of such relationships. Trails B and DST are important indicators of cognitive function (Lezak, 1995) and Trails B provides a robust examination of cognitive domains relevant to the management of medications, household, and medical care for oneself and one’s spouse. (c) Matching on five variables (age, gender, income, race and education) and controlling for IQ (Shipley Vocabulary and Abstraction scores) enhanced our ability to make inferences about CG status and cognitive function in relatively small nonrandom samples. Moreover, we controlled for HRT and psychoactive drugs and the groups did not differ on health variables (e.g., diabetes, stroke, hypertension, coronary heart disease [CHD]).
Future research should examine these relationships using larger more representative samples. Interventions can be used to assess whether reductions in burden are accompanied by reductions in SCPs. We know of only one study that has observed clinically significant improvements in cognition after reducing CG distress. The study sample was small, but the intervention was grounded in research on cognition and CG distress (Mackenzie, Wiprzycka, Khatri, & Cheng, 2013) and can serve as a model for future work. Because DST and Trails B were associated with SCPs in NCGs, but not in CGs, researchers/clinicians should investigate whether CGs have poorer self-monitoring than NCGs, as this is important for tracking behaviors and may be relevant to household function and safety. SCPs are often used to screen for overall function in older adults, but such reports may have a different meaning depending on one’s CG status. Finally, research showing biological markers as mediators of CG stress and greater cognitive problems should be useful in understanding the relationships observed here (Corrêa et al., 2015; Vitaliano et al., 2005), but such analyses would have added another layer of complexity to these analyses. In conclusion, both SCPs (Jonker et al., 2000; Schofield et al., 1997; Wilson & Evans, 1996) and poorer objective cognitive deficits (Cahn-Weaver, Boyle, & Malloy, 2002) may predict future long-term CG disability. As such, research needs to further determine the importance of SCPs and objective cognitive deficits in predicting long-term CG and care recipient health.
Funding
This study was supported by grants to P.P.V. from National Institute of Mental Health, R01 National Institute of Mental Health (RO1-MH57663), and National Institute of Aging (R03 AG023629).
References
Author notes
Decision Editor: Rachel Pruchno, PhD