-
PDF
- Split View
-
Views
-
Cite
Cite
Carl Sellgren, Thomas Frisell, Paul Lichtenstein, Mikael Landèn, Johan Askling, The Association Between Schizophrenia and Rheumatoid Arthritis: A Nationwide Population-Based Swedish Study on Intraindividual and Familial Risks, Schizophrenia Bulletin, Volume 40, Issue 6, November 2014, Pages 1552–1559, https://doi.org/10.1093/schbul/sbu054
Close - Share Icon Share
Abstract
Numerous studies have reported a reduced risk of rheumatoid arthritis (RA) in schizophrenia. The mechanisms are unknown, but recent genome-wide association studies of schizophrenia have shown strong associations with markers spanning the major histocompatibility complex region, indicating a possible role for adaptive immunity also in schizophrenia. In this population-based cohort study, we assess the associations between RA and schizophrenia and the extent to which any observed associations are specific to RA/schizophrenia. We then extend the assessments per RA subtype and to risks in first-degree relatives. The study population included every individual identified in the Swedish Population Register born in Sweden between 1932 and 1989. The risk for RA in schizophrenia was significantly decreased (hazard ratio [HR] = 0.69, 95% CI = 0.59–0.80), but similar reductions were noted for osteoarthritis (a noninflammatory joint disorder) and ankylosing spondylitis (a non-RA inflammatory disorder). Comparable associations were seen in schizoaffective subjects while no significant associations were observed in bipolar disorder. Overall, first-degree relatives of schizophrenia patients were not at reduced risk of RA, but the risk for seronegative RA was significantly decreased in children and siblings of schizophrenia probands (HR = 0.13, 95% CI = 0.02–0.95 and HR = 0.67, 95% CI = 049–0.92, respectively). In conclusion, our intraindividual analyses suggest that differential misclassification bias is an important factor for the observed inverse association and emphasize the need of optimized care-provision for nonpsychiatric symptoms in schizophrenia patients. Our familial analyses indicted the possibility of an inverse coinheritance of schizophrenia and seronegative RA.
Introduction
Rheumatoid arthritis (RA) is an immune-mediated chronic inflammatory joint disease caused by a combination of genetic and environmental factors. The overall heritability of RA is estimated to be around 50%, and the major histocompatibility complex (MHC) region is the single most important genetic risk locus.1 In schizophrenia, there is also accumulating evidence for a role of immune signaling.2 For example, an increased peripheral concentration of IL-1 receptor antagonist (IL-1Ra) has been reported in patients with schizophrenia,3 and knockout mice lacking IL-1Ra develop spontaneous joint inflammation.4 Recent genome-wide association studies of schizophrenia have also found strong associations with markers spanning the MHC region,5 indicating a possible role for adaptive immunity also in schizophrenia.
Numerous epidemiological studies have described an inverse association between RA and schizophrenia.6–9 The association remains poorly understood but has been suggested to be due to a shared immune-related etiology,10 possibly attributed to genetic factors.7,8 The majority of these studies have assessed the risk of RA in patients with schizophrenia. Because the typical age of onset is higher in RA than in schizophrenia, such assessments may be biased by underreporting or underdiagnosis of RA in patients with severe mental disorders.7 An alternative approach that would largely circumvent such differential misclassification bias, and also reveal potential common genetic determinants, would be to assess the risk of RA in first-degree relatives of probands with schizophrenia, and vice versa. Furthermore, “RA” is a criteria-based diagnosis that contains etiologically distinct subtypes, eg, as defined by the presence of serological markers. Recent studies of RA also suggest that seronegative and seropositive RA, the latter defined by presence of rheumatoid factor (RF) and/or anticitrullinated peptide/protein antibodies (ACPA), have different etiologies and genetic architectures.11 The MHC is most strongly associated with ACPA-positive RA.12–14 Most, but not all, ACPA-positive patients are also RF-positive, and it is estimated that 50%–80% of all individuals with RA have RF, ACPA, or both.15 Hence, a comprehensive assessment of the association between RA and schizophrenia needs to take RA subtype into account.
So far, attempts to study familial associations between RA and schizophrenia have not provided conclusive results, mainly because of small samples yielding limited statistical precision. Of the 4 existing studies—none of which were primarily designed to assess a familial association between schizophrenia and RA—1 study, based on interviews with mothers of psychotic patients (n = 101), found that RA was significantly less common in the mothers, but no association with RA in the patients’ first-degree relatives overall was observed.16 In a second, larger study based on national registries, parents of schizophrenia patients had a higher than expected prevalence of RA,17 although this association did not reach statistical significance. In a third study, based on 515 RA patients and 769 controls, 23 subjects had a family history of schizophrenia, and the observed relative risk of approximately 2 did not reach significance.18 However, in the same study, patients with RA characterized by the presence of ACPA were at increased risk of having a first-degree relative with schizophrenia, whereas no such association was seen in ACPA-negative RA. The fourth and most recent study explored the risk of autoimmune diseases, including RA, in individuals with a family history of schizophrenia spectrum disorder using Danish nationwide registers.9 A total of 294 out of 9697 individuals with a register-based diagnosis of RA had a parent or sibling with a diagnosis of schizophrenia, but the risk was not different from that of the general population.
In this study, we aimed to assess the relative risk of RA in patients with schizophrenia and in their first-degree relatives (children, siblings, and parents), using a sufficiently large study population and—to the extent possible—assess these risks in relation to seropositive and seronegative RA (as defined by the 10th revision of the International Classification of Diseases [ICD-10]).19 Also in contrast to previous studies, we assessed the risk of schizophrenia in first-degree relatives of RA probands. To gauge effects primarily related to diagnostic intensity, we also assessed risks of RA in patients with schizoaffective and bipolar disorder (BD). We furthermore investigated the risks of noninflammatory musculoskeletal diseases (osteoarthritis [OA]) and inflammatory non-RA diseases (ankylosing spondylitis [AS]) in patients with these 3 major psychotic disorders and in their first-degree relatives.
Methods
Data Sources Used
All residents in Sweden receive a 10-digit personal identification number, which is consistent throughout all national registries, and hence make register linkage possible.20 The National Patient Register (NPR; held at The National Board of Health and Welfare) contains information on inpatient care since 1964. Diagnoses are reported using the ICD.19,21,22 Every record has an admission and discharge date, together with the main diagnosis and secondary diagnoses. Since 2001, the NPR registers corresponding data from outpatient visits in specialized nonprimary care. The nationwide Multi-Generation Register (MGR) (Statistics Sweden) holds information on first-degree relatives of Swedish residents born from 1932 and onwards who were alive in or after 1961. In addition to the NPR and MGR, we also obtained data from the Migration, Cause of Death, and Total Population Register at Statistics Sweden.
Source Population
The study population was defined as every individual identified in the Swedish Population Register born in Sweden between January 1932 and December 1989. Immigrants were included if less than 18 years old when registered in Sweden or if they immigrated as an adult before January 1958. The study was approved by the Ethics committee at Karolinska Institutet, Stockholm, Sweden.
Identification of Schizophrenia and Other Psychiatric Diagnoses
The inpatient component of the NPR contains all public psychiatric inpatient admissions in Sweden since 1973 (for outpatient care since 2001). Subjects with schizophrenia were identified based on at least 1 visit listing schizophrenia in the inpatient component of the NPR (from 1973 through 2009) or the outpatient nonprimary component (from 2001 through 2009). For comparison reasons, we also identified subjects with schizoaffective disorders and BD. Identification of subjects with schizoaffective disorder was also based on at least 1 visit in the NPR listing this diagnosis, while a previously validated algorithm, based on 2 or more separate registrations in the NPR, was used to identify BD subjects.23 The diagnostic structure was nonhierarchical, meaning that a subject could have more than one diagnosis. All diagnostic codes were retrieved from the NPR, and the ICD codes used are listed in Supplementary Data.
Identification of RA and Other Musculoskeletal Diagnoses
Subjects with RA were identified based on at least 1 visit in the NPR listing a RA diagnosis from 1997 and through 2009. Start of follow-up was in 1997 when ICD-10 was introduced in Sweden (previous ICD classifications do not separate seropositive and seronegative RA). To assess effects related to the RA diagnosis per se rather than effects related to musculoskeletal symptoms or access to (rheumatology) care, we also identified all subjects with at least 1 visit in the NPR listing a diagnosis of OA or AS (Supplementary Data).
Study Design
We performed cohort analyses using prospectively recorded register data. In the intraindividual analyses, we assessed the association between schizophrenia (exposure) and risk of RA (outcome). In the familial analyses, we assessed the association between having a first-degree relative with schizophrenia (exposure) and risk of RA overall and by subtype (outcome). We also performed analyses of the risk of schizophrenia in first-degree relatives of patients with RA overall and by subtype. For comparison reasons, the same analyses were also run for schizoaffective disorder and BD (instead of schizophrenia), and for AS and OA (instead of RA).
Statistical Analyses
We first analyzed intraindividual risks of developing RA overall and by subtype, AS, and OA following a diagnosis of schizophrenia, schizoaffective disorder, or BD, respectively. We used Cox regression models with attained age as time scale, and all analyses were adjusted for sex. All subjects in the study population were followed from birth (or January 1, 1997 if born before this date). Exposure was treated as a time-varying covariate, such that a person was considered as unexposed before the date of the first psychiatric diagnosis, and as exposed thereafter. Follow-up ended with first diagnosis of the rheumatological outcome under study, death, emigration, or on December 31, 2009. Regarding risk of RA overall, we also performed additional analyses using the full study period (start of follow-up in 1973). In a sensitivity analysis of RA by subtype, we excluded patients with an RA overall diagnosis before start of follow-up in 1997.
We then assessed the risk of RA (overall and by subtype), AS, and OA in first-degree relatives of patients with the psychotic disorder under study compared to that of first-degree relatives of nonpatients. Conversely, we also assessed the risk of the psychotic disorder under study in first-degree relatives of patients with these rheumatological disorders. To verify that the study design was able to identify the anticipated disease-specific familial associations, we performed additional analyses to assess risk of the disorder under study in first-degree relatives of individuals with this disorder. We used Cox regression with robust sandwich CIs (attained age as time scale). Exposure was time varying, here meaning that first-degree relatives were considered unexposed up until the date of first occurrence of the proband’s first diagnosis, and as exposed thereafter. Follow-up began at birth (or January 1, 1997) and ended with first occurrence of the outcome diagnoses, end-of-follow-up (December 31, 2009), death, or emigration. All analyses were adjusted for sex.
Results
We identified 5 981 124 individuals born in Sweden and 432 559 individuals born outside Sweden, between 1932 and 1989. In total, 31 193 unique individuals with a diagnosis of schizophrenia were identified in the NPR. Through linkage with the MGR, 74 260 first-degree relatives of schizophrenia probands were identified (table 1). A total of 10 676 individuals with schizoaffective disorder and 34 744 individuals with BD were identified, together with 27 112 first-degree relatives of probands with schizoaffective disorder and 97 528 first-degree relatives of probands with BD. The median age (interquartile range) at first entry for schizophrenia patients was 44 years (19); for schizoaffective disorder patients, 41 years (19); and for BD patients, 38 years (20). The attained age of first-degree relatives (children, siblings, parents) at start/end of follow-up was similar to the corresponding group of first-degree relatives of schizophrenia probands (data not shown).
Background Characteristics for Schizophrenia Patients and Their Relatives
| . | Index Patients With Schizophrenia . | Children of Patients With Schizophrenia . | Siblings of Patients With Schizophrenia . | Parents of Patients With Schizophrenia . |
|---|---|---|---|---|
| Number | 31 193 | 13 948 | 44 675 | 15 637 |
| Males/females, % | 59/41 | 52/48 | 51/49 | 42/58 |
| Year of birth, median (IQR)a | 1955 (20)a | 1973 (15) | 1954 (18) | 1941 (11)a |
| Calendar year of first entry, median (IQR) | 1997 (5) | 1997 (4) | 1997 (5) | 1998 (8) |
| Age at first entry, median (IQR) | 44 (19) | 26 (15)/35 (15)b | 44 (18)/54 (18)b | 58 (10)/67 (11)b |
| Person-time of follow-up evaluation (person-years) | 290 227 | 127 824 | 415 513 | 126 447 |
| . | Index Patients With Schizophrenia . | Children of Patients With Schizophrenia . | Siblings of Patients With Schizophrenia . | Parents of Patients With Schizophrenia . |
|---|---|---|---|---|
| Number | 31 193 | 13 948 | 44 675 | 15 637 |
| Males/females, % | 59/41 | 52/48 | 51/49 | 42/58 |
| Year of birth, median (IQR)a | 1955 (20)a | 1973 (15) | 1954 (18) | 1941 (11)a |
| Calendar year of first entry, median (IQR) | 1997 (5) | 1997 (4) | 1997 (5) | 1998 (8) |
| Age at first entry, median (IQR) | 44 (19) | 26 (15)/35 (15)b | 44 (18)/54 (18)b | 58 (10)/67 (11)b |
| Person-time of follow-up evaluation (person-years) | 290 227 | 127 824 | 415 513 | 126 447 |
Note: IQR = interquartile range. All subjects were identified in a Swedish nationwide population-based cohort with earliest start of follow-up in 1997.
aSchizophrenia patients without information regarding one or both parents were also included. This was more common for individuals born early in the study period. Median paternal/maternal age can hence not be correctly estimated from this data as parents of these individuals do not contribute to median year of birth among parents.
bAttained age at start/stop follow-up.
Background Characteristics for Schizophrenia Patients and Their Relatives
| . | Index Patients With Schizophrenia . | Children of Patients With Schizophrenia . | Siblings of Patients With Schizophrenia . | Parents of Patients With Schizophrenia . |
|---|---|---|---|---|
| Number | 31 193 | 13 948 | 44 675 | 15 637 |
| Males/females, % | 59/41 | 52/48 | 51/49 | 42/58 |
| Year of birth, median (IQR)a | 1955 (20)a | 1973 (15) | 1954 (18) | 1941 (11)a |
| Calendar year of first entry, median (IQR) | 1997 (5) | 1997 (4) | 1997 (5) | 1998 (8) |
| Age at first entry, median (IQR) | 44 (19) | 26 (15)/35 (15)b | 44 (18)/54 (18)b | 58 (10)/67 (11)b |
| Person-time of follow-up evaluation (person-years) | 290 227 | 127 824 | 415 513 | 126 447 |
| . | Index Patients With Schizophrenia . | Children of Patients With Schizophrenia . | Siblings of Patients With Schizophrenia . | Parents of Patients With Schizophrenia . |
|---|---|---|---|---|
| Number | 31 193 | 13 948 | 44 675 | 15 637 |
| Males/females, % | 59/41 | 52/48 | 51/49 | 42/58 |
| Year of birth, median (IQR)a | 1955 (20)a | 1973 (15) | 1954 (18) | 1941 (11)a |
| Calendar year of first entry, median (IQR) | 1997 (5) | 1997 (4) | 1997 (5) | 1998 (8) |
| Age at first entry, median (IQR) | 44 (19) | 26 (15)/35 (15)b | 44 (18)/54 (18)b | 58 (10)/67 (11)b |
| Person-time of follow-up evaluation (person-years) | 290 227 | 127 824 | 415 513 | 126 447 |
Note: IQR = interquartile range. All subjects were identified in a Swedish nationwide population-based cohort with earliest start of follow-up in 1997.
aSchizophrenia patients without information regarding one or both parents were also included. This was more common for individuals born early in the study period. Median paternal/maternal age can hence not be correctly estimated from this data as parents of these individuals do not contribute to median year of birth among parents.
bAttained age at start/stop follow-up.
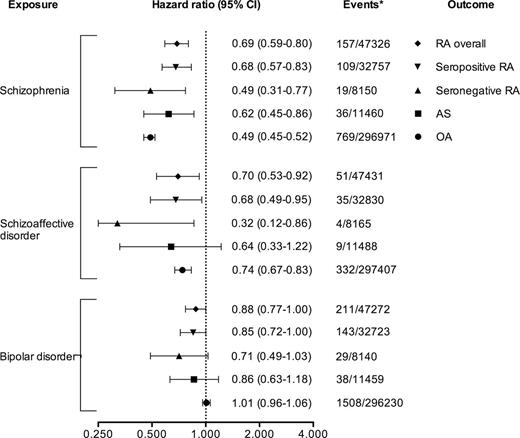
Risk of RA Overall and by Subtype in Individuals With Schizophrenia
Schizophrenia patients had a significantly reduced risk of receiving a subsequent diagnosis of RA overall (hazard ratio [HR] = 0.69, 95% CI = 0.59–0.80), but also for AS (HR = 0.62, 95% CI = 0.45–0.86) as well as OA (HR = 0.49, 95% CI = 0.45–0.52). When RA was assessed by subtype, the lowest point estimate was observed for seronegative RA (seropositive RA: HR = 0.68, 95% CI = 0.57–0.83 vs seronegative RA: HR = 0.49, 95% CI = 0.31–0.77). Similar associations were seen in individuals with schizoaffective disorder, while in BD, the risk of later RA, AS, or OA was not different from that of the general population. All relative risks are presented in figure 1. The inverse association between schizophrenia and RA displayed no obvious trend by attained age (Supplementary Data), and the result of a sensitivity analysis requiring 2 diagnoses to be classified as having schizophrenia was similar regarding the risk of later RA (HR = 0.72, 95% CI = 0.61–0.86). Excluding patients with a RA diagnosis before start of follow-up in 1997 had no major influence on the results (data not shown), and using the full study period from 1973, the relative risk of RA overall in schizophrenia patients was similar to the analysis with start of follow-up in 1997 (HR = 0.76, 95% CI = 0.66–0.89; Supplementary Data). Additional analyses stratified by sex, birth year, and birth country (categorized as “Sweden,” “Scandinavia excluding Sweden,” and “Other”) were also performed but without any substantial impact on the relative risks (RA overall: HR = 0.70, 95% CI = 0.60–0.82; seropositive RA: HR = 0.70, 95% CI = 0.58–0.82; and seronegative RA: HR = 0.50, 95% CI = 0.32–0.79). Sex-stratified relative risks of developing RA overall and by subtype can also be found in Supplementary Data.
Relative risks of developing rheumatoid arthritis (RA), overall and by subtype, for patients with schizophrenia. For comparison reasons, relative risks of receiving a subsequent diagnosis of ankylosing spondylitis (AS) and osteoarthritis (OA) are also shown. All data are from a Swedish nationwide population-based cohort with earliest start of follow-up in 1997. *Events among exposed/unexposed.
The Association Between Schizophrenia and Rheumatoid Arthritis
Disease-specific familial risks were similar to what has been shown in previous studies.11,24 A family history of RA was associated with a tripled risk for RA (HR = 3.4, 95% CI = 3.2–3.6), and a family history of schizophrenia was associated with an 8-fold risk of schizophrenia (HR = 8.2, 95% CI = 7.6–8.8).
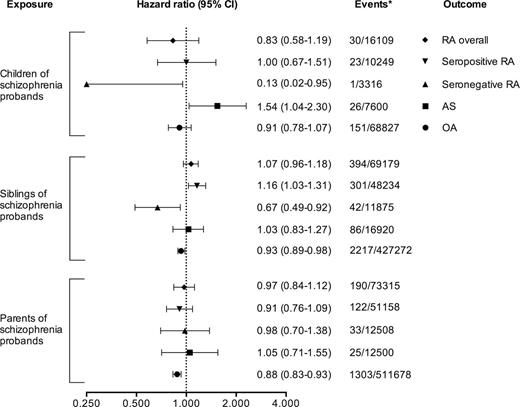
Risk of RA Overall and by Subtype in First-Degree Relatives of Individuals With Schizophrenia
No significantly increased or decreased risk of receiving a diagnosis of RA overall could be detected in children, siblings, or parents of schizophrenia probands (HR = 0.83, 95% CI = 0.58–1.19; HR = 1.07, 95% CI = 0.96–1.18; and HR = 0.97, 95% CI = 0.84–1.12, respectively). For seropositive RA, we observed a slightly increased risk in siblings of schizophrenia probands (HR = 1.16, 95% CI = 1.03–1.31), while the risk in children (HR = 1.00, 95% CI = 0.67–1.51) and parents (HR = 0.91, 95% CI = 0.76–1.09) were comparable with that of the general population. For seronegative RA, we noted decreased risks in siblings of schizophrenia probands (HR = 0.67, 95% CI = 0.49–0.92) and in children (HR = 0.13, 95% CI = 0.02–0.95), while the risk in parents did not differ significantly from that of the general population (HR = 0.98, 95% CI = 0.70–1.38). Apart from a small yet statistically significant reduction in risk of OA in siblings and parents of patients with schizophrenia (HR = 0.93, 95% CI = 0.89–0.98 and HR = 0.88, 95% CI = 0.83–0.93, respectively), no inverse associations with schizophrenia were noted for OA or AS. All relative risks are presented in figure 2. For schizoaffective disorder or BD, no associations were observed with relatives’ risk of RA, neither for RA overall nor per RA subtype (Supplementary Data and Supplementary Data). We also performed the same analyses only counting exposure from January 1, 1997 and onwards, ie, probands diagnoses before this date were not taken into account regarding exposure of the first-degree relatives, with similar results (data not shown).
Relative risks of developing rheumatoid arthritis (RA), overall and by subtype, for first-degree relatives of probands with schizophrenia. For comparison reasons, relative risks of receiving a subsequent diagnosis of ankylosing spondylitis (AS) and osteoarthritis (OA) are also shown. All data are from a Swedish nationwide population-based cohort with earliest start of follow-up in 1997. *Events among exposed/unexposed.
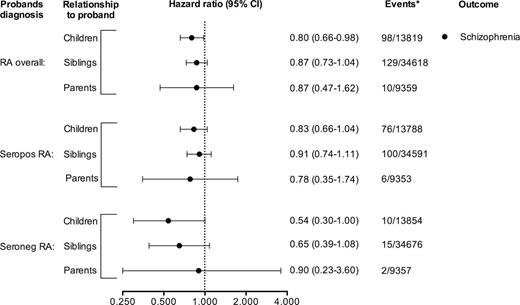
Risk of Schizophrenia in First-Degree Relatives of Individuals With RA by Subtype
Among first-degree relatives of RA probands, all point estimates for schizophrenia were below unity. The decreased risk, however, only reached statistical significance in children of probands with RA overall (HR = 0.80, 95% CI = 0.66–0.98). In children of probands with seronegative RA, the decreased risk also nearly reached significance (HR = 0.54, 95% CI = 0.30–1.00) (figure 3). For schizoaffective disorder and BD, we only observed a significantly increased risk of BD in children of patients with RA overall (HR = 1.19, 95% CI = 1.03–1.37) and an increased risk of schizoaffective disorder in children of patients with seronegative RA (HR = 1.79, 95% CI = 1.04–3.09), while all other risks were comparable with that of the general population (Supplementary Data and Supplementary Data).
Relative risks of developing schizophrenia for first-degree relatives of probands with rheumatoid arthritis (RA) overall and by subtype. All data are from a Swedish nationwide population-based cohort with earliest start of follow-up in 1997. *Events among exposed/unexposed.
Discussion
In line with previous reports, we found a significantly decreased risk for RA in subjects with a history of schizophrenia. The risks for OA, a noninflammatory joint disorder, and for AS, a non-RA inflammatory disorder, were however similarly decreased, suggesting that a substantial part of the observed inverse association between RA and schizophrenia may be due to other factors than disease-specific biology. This finding is consistent with the results from a prior study reporting comparably decreased incidences of degenerative musculoskeletal disorders and RA in schizophrenia patients.7 Further, we found no associations between BD and RA despite a previous study’s report of a shared polygenic component of schizophrenia and BD of 68%.25 In light of the more severe loss of function in schizophrenia and schizoaffective disorder patients in comparison to BD patients,26 this is compatible with differential misclassification bias in the observed inverse association between schizophrenia and RA. Interestingly, patients with schizophrenia or related psychosis have been reported to be at increased risk of other autoimmune diseases with a different symptomatology.9 Clearly, potential effect modulation due to underreporting/underdiagnosis depends on types of symptoms. Of clinical importance, these data however emphasize the need of assessing and being aware of nonpsychiatric symptoms in schizophrenia patients.
In the analyses of first-degree relatives of schizophrenia probands, we observed no significant associations with RA overall. However, analyzing RA risk by subtype, we observed a significantly decreased risk for seronegative RA in children (HR = 0.13) and siblings (HR = 0.67) of schizophrenia probands. Correspondingly, the lowest point estimate for schizophrenia was observed in relatives of seronegative RA patients (HR = 0.54), and the lowest point estimate among schizophrenia patients was also observed for seronegative RA (HR = 0.49). This was unexpected, as the strongest link with MHC has been found for seropositive RA. On the other hand, because seronegative RA is considerably less common than seropositive RA, and may in itself be a heterogeneous entity, less is known about the etiology and link to MHC in this RA subset. In BD, we observed no significantly decreased risk for RA overall, or per subgroup. Molecular genetics studies also suggest a greater MHC involvement in schizophrenia compared with BD.27 The strongest single nucleotide polymorphism association with schizophrenia is also located in the extended MHC,5 but the complexity of the region, including strong linkage disequilibrium (LD) and a high gene density, has complicated efforts to identify causal variations. The classical MHC I haplotype HLA-B*08 has been associated with a decreased risk of schizophrenia.27–29 This motif is reported to predispose for RA,30 although hitherto only shown in ACPA-positive RA patients. Both MHC class II and I are also involved in synaptic plasticity,31.32 and a direct role for these molecules in the pathophysiology of schizophrenia is therefore possible. Importantly, the schizophrenia locus is located in a region with high LD and extends over several Mb.5 “Tagging” could hence explain associations between classical motifs and schizophrenia. Our epidemiological data also indicate a decreased risk for seronegative RA but not for seropositive RA (as defined by ICD-10 codes). Motifs foremost associated with ACPA, such as the shared epitopes (SEs) alleles of HLA-DRB1,1,30 are therefore unlikely to explain the familial associations as the vast majority of ACPA-positive RA patients have seropositive RA.
The strengths of our data include the national coverage and the fact that this is the largest study of intraindividual and interfamilial associations between schizophrenia and RA. The size of our study population in combination with the number of events during follow-up and the duration of follow-up provided sufficient power to detect even moderate differences in risks overall and stratified RA subtype. The use of outpatient data, in combination with the hospital discharge register, also diminished the risk of bias related to hospitalization rather than the studied disease per se.
A limitation of this study is the use of diagnostic information that depends on data provided from national registries. Nevertheless, Swedish register discharge diagnoses of schizophrenia have shown a high concordance with diagnoses based on semi-structured interviews.33 Similarly, register-based RA diagnoses have a high diagnostic accuracy and correspond to a typical RA prevalence.34 Another drawback of the population-based approach is the limited data regarding potential confounders. For example, smoking is more common in schizophrenia patients than in their first-degree relatives,35 and the risk of developing ACPA-positive RA is associated with a gene-environment interaction between smoking and the HLA-DRB1 SE alleles.36 Hence, it is possible that the estimated relative risk for seropositive RA in schizophrenia patients (but perhaps not in their first-degree relatives) was inflated by uncontrolled confounding from smoking. Of note, we also observed a significantly increased risk of seropositive RA in schizophrenia siblings. However, confounding by smoking could hardly explain the decreased risks for seronegative RA in schizophrenia patients and their first-degree relatives. Less is known about other environmental triggers for RA, and few other robust gene-environment interactions have been reported.1,15 Because the heritability of RA is estimated to be around 50%, leaving the remaining part to environment and interactions, further uncontrolled confounding cannot be excluded. Perinatal factors have for example been implicated in schizophrenia,37 and may also play a role in RA,1 but are still sparsely studied, at least if also considering timing of exposure. Finally, stratification by ACPA serostatus, in combination with RF status (ie, ICD-10), would have further improved the specificity of the heterogeneous RA phenotype.
In conclusion, our analyses suggest that underreporting, or underdiagnosis, is an important factor for the inverse intraindividual association of schizophrenia and RA. However, an unexpected inverse association between seronegative RA and schizophrenia due to genetic causation, including unknown gene-environment interactions, cannot be ruled out. Taken together, further molecular genetic studies of the association between RA and schizophrenia should include stratification by RA subtype and focus on the unique genetic cause of schizophrenia rather than the common genetic cause of schizophrenia and BD.
Funding
This work was supported by the Swedish Medical research Council (K2011-61X -14647-09-3, K2010-61X-21569-01-1, and 2010-61P-21568-01-4 to M.L.); the Swedish Foundation for Strategic Research; and from the public private COMBINE research consortium.
Acknowledgments
We are grateful to Dr Leonid Padyukov (Rheumatology Unit, Department of Medicine at the Karolinska University Hospital in Solna) and Dr Elizabeth Arkema (Clinical Epidemiology Unit, Department of Medicine Solna, Karolinska Institutet) for their valuable comments and suggestions in the drafting of the manuscript. The sponsors had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References