-
PDF
- Split View
-
Views
-
Cite
Cite
Graham Blackman, Ebenezer Oloyede, Mark Horowitz, Robert Harland, David Taylor, James MacCabe, Philip McGuire, Reducing the Risk of Withdrawal Symptoms and Relapse Following Clozapine Discontinuation—Is It Feasible to Develop Evidence-Based Guidelines?, Schizophrenia Bulletin, Volume 48, Issue 1, January 2022, Pages 176–189, https://doi.org/10.1093/schbul/sbab103
Close - Share Icon Share
Abstract
Clozapine is the only antipsychotic that is effective in treatment-resistant schizophrenia. However, in certain clinical situations, such as the emergence of serious adverse effects, it is necessary to discontinue clozapine. Stopping clozapine treatment poses a particular challenge due to the risk of psychotic relapse, as well as the development of withdrawal symptoms. Despite these challenges for the clinician, there is currently no formal guidance on how to safely to discontinue clozapine. We assessed the feasibility of developing evidence-based recommendations for (1) minimizing the risk of withdrawal symptoms, (2) managing withdrawal phenomena, and (3) commencing alternatives treatment when clozapine is discontinued. We then evaluated the recommendations against the Appraisal of Guidelines for Research and Evaluation (AGREE) II criteria. We produced 19 recommendations. The majority of these recommendation were evidence-based, although the strength of some recommendations was limited by a reliance of studies of medium to low quality. We discuss next steps in the refinement and validation of an evidence-based guideline for stopping clozapine and identify key outstanding questions.
Introduction
Clozapine is a dibenzodiazepine antipsychotic that was first developed in 1959.1 It is the only effective treatment for patients with schizophrenia who are treatment-resistant,2 and the most effective treatment for schizophrenia more generally.3–5 There are certain circumstances, however, where it is necessary to stop clozapine with estimates of discontinuation ranging between 16% and 66%.6–15 There are several reasons to stop clozapine, however they can be broadly divided into indications leading to either emergency, or elective discontinuation (table 1). Emergency discontinuation of clozapine is typically indicated when patients experience potentially life-threatening adverse effects, such as agranulocytosis, myocarditis, or neuroleptic malignant syndrome.16 In some situations, clozapine can be reinitiated once the clinical concern has resolved, or has been mitigated. Examples where clozapine can be discontinued electively include intolerable (but not immediately life-threatening) adverse effects, lack of treatment response, patient preference, and poor adherence. There are also some instances where clozapine can be discontinued after full recovery has been achieved.17,18 In contrast to stopping clozapine in an emergency, elective discontinuation usually allows a period of consultation, information gathering, and preparation as well as the option of stopping clozapine more gradually.
Reasons for Discontinuation or Interruption of Clozapine
| Emergency . | Elective . |
|---|---|
| Seizures | Adverse effects (eg, sedation, tachycardia, dizziness, hypersalivation, nausea and vomiting)b |
| Agranulocytosis (overlaps with leukopenia and neutropenia) | Inadequate adherence to treatment or monitoring requirementsc |
| Red result on haematological monitoringa | Lack of clinical efficacyd |
Neuroleptic malignant syndrome Myocarditis Intestinal obstruction QTc prolongation (>500 ms) | Patient preference (eg, patient unable to tolerate blood monitoring or reports that the adverse effects outweigh the beneficial effects) |
| Cardiomyopathy |
| Emergency . | Elective . |
|---|---|
| Seizures | Adverse effects (eg, sedation, tachycardia, dizziness, hypersalivation, nausea and vomiting)b |
| Agranulocytosis (overlaps with leukopenia and neutropenia) | Inadequate adherence to treatment or monitoring requirementsc |
| Red result on haematological monitoringa | Lack of clinical efficacyd |
Neuroleptic malignant syndrome Myocarditis Intestinal obstruction QTc prolongation (>500 ms) | Patient preference (eg, patient unable to tolerate blood monitoring or reports that the adverse effects outweigh the beneficial effects) |
| Cardiomyopathy |
aDefined within the UK as an absolute neutrophil count <1.5 × 109 or a white cell count <3 × 109 in the general population (cut-off lowered by 0.5 × 109 in patients with benign ethnic neutropenia).19,20
bStrategies to counteract adverse effects should be employed first.
cStrategies to maximise adherence should be employed first.
dStrategies to maximise efficacy should be employed first.
Reasons for Discontinuation or Interruption of Clozapine
| Emergency . | Elective . |
|---|---|
| Seizures | Adverse effects (eg, sedation, tachycardia, dizziness, hypersalivation, nausea and vomiting)b |
| Agranulocytosis (overlaps with leukopenia and neutropenia) | Inadequate adherence to treatment or monitoring requirementsc |
| Red result on haematological monitoringa | Lack of clinical efficacyd |
Neuroleptic malignant syndrome Myocarditis Intestinal obstruction QTc prolongation (>500 ms) | Patient preference (eg, patient unable to tolerate blood monitoring or reports that the adverse effects outweigh the beneficial effects) |
| Cardiomyopathy |
| Emergency . | Elective . |
|---|---|
| Seizures | Adverse effects (eg, sedation, tachycardia, dizziness, hypersalivation, nausea and vomiting)b |
| Agranulocytosis (overlaps with leukopenia and neutropenia) | Inadequate adherence to treatment or monitoring requirementsc |
| Red result on haematological monitoringa | Lack of clinical efficacyd |
Neuroleptic malignant syndrome Myocarditis Intestinal obstruction QTc prolongation (>500 ms) | Patient preference (eg, patient unable to tolerate blood monitoring or reports that the adverse effects outweigh the beneficial effects) |
| Cardiomyopathy |
aDefined within the UK as an absolute neutrophil count <1.5 × 109 or a white cell count <3 × 109 in the general population (cut-off lowered by 0.5 × 109 in patients with benign ethnic neutropenia).19,20
bStrategies to counteract adverse effects should be employed first.
cStrategies to maximise adherence should be employed first.
dStrategies to maximise efficacy should be employed first.
Discontinuing clozapine in patients with schizophrenia is associated with an increased risk of relapse of psychotic symptoms.21,22 This is particularly problematic in patients with treatment-resistant schizophrenia, as other antipsychotic drugs are unlikely to be effective. Furthermore, clozapine can be associated with a range of withdrawal symptoms. These include catatonia, sleep disturbance, confusion, autonomic dysfunction, neuromuscular and gastrointestinal symptoms, as well as psychotic symptoms that can be worse than the underlying condition.22,23,24 A recent international pharmacovigilance study found that withdrawal symptoms account for a substantial proportion of all clozapine-related adverse drug reactions.25
Clinicians may be reluctant to initiate treatment with clozapine due to the concern that once treatment has started, discontinuation is hazardous and difficult to manage. Unlike starting clozapine, there are very few recommendations around how to safely discontinue clozapine.26 The recognition that abrupt discontinuation can result in withdrawal symptoms brought about a recommendation for gradual tapering in 199527 and subsequent guidance documents have recommended that discontinuation takes place over one to two weeks.16,28 However, there is evidence to suggest that this taper period may be too short. Furthermore, there is an absence of guidance on the wider aspects of clozapine discontinuation, such as managing withdrawal symptoms and selecting alternative antipsychotic treatment. Simple, evidence-based recommendations around the safe discontinuation of clozapine would help to address this issue. We therefore sought to assess the feasibility of developing guidelines that cover these topics based on the available evidence.
Methods
Procedure
We assessed the feasibility of developing clinical guidelines for mental health professionals managing patients with schizophrenia who are being discontinued on clozapine. The use of clozapine for other indications, such as psychosis in Parkinson’s disease were not considered. Provisional guidelines were initially developed by psychiatrists and pharmacists at the South London and Maudsley NHS Foundation Trust with expertise in managing patients on clozapine. They were then reviewed by a patient advisory group and revised accordingly. Authors then evaluated the guideline against the Appraisal of Guidelines for Research and Evaluation (AGREE) II criteria.29,30
Evidence Collation and Synthesis
The published literature up to April 2020 was independently reviewed by two authors (G.B. and E.O.) using the search terms in PubMed: clozapine AND discontinuation OR withdrawal OR relapse OR rapid onset psychosis OR withdrawal-associated psychosis OR dopamine supersensitivity OR tardive psychosis OR supersensitivity psychosis. In addition, current guidelines on the management of schizophrenia were reviewed, including the World Federation of Societies of Biological Psychiatry (WFSBP),31 British Association for Psychopharmacology (BAP),32 German Association for Psychiatry, Psychotherapy and Psychosomatics (DGPPN),33 Royal Australian and New Zealand College of Psychiatrists (RANZCP),34 National Institute for Health and Care Excellence Clinical Guidelines 178 (NICE),35 Scottish Intercollegiate Guidelines Network (SIGN),36 American Psychiatric Association (APA),37 Schizophrenia Patient Outcomes Research Team (PORT),38 and the Maudsley Prescribing guidelines.39
Assessing the Strength of Evidence and Recommendation
For each recommendation, the highest level of directly relevant evidence was assessed following the North of England evidence-based guideline development project categories of evidence for causal relationships.40 Evidence was graded I (evidence from randomized controlled trials) to IV (evidence from expert committee or respected authority). The strength of each recommendation was also rated, based on the relevance and grade of the supporting evidence, from A (directly based on category I evidence) to D (directly based on category IV evidence or extrapolated recommendation from category I, II, or III evidence). The evidence for any intervention was also weighed against potential risks and opportunity costs.40 In the absence of relevant evidence, consensus-based recommendations were developed.
Withdrawal-Associated Psychosis
The discontinuation of clozapine can be associated with a rapid onset of psychotic symptoms. The term “withdrawal-associated psychosis” has been used to describe the prompt emergence of psychosis that occurs in a subgroup of patients when antipsychotics are discontinued.41 The prevalance of withdrawal-associated psychosis following abrupt discontinuation of clozapine is estimated to be as high as 20%.42 Early prospective studies on clozapine cessation, without switching to an alternative antipsychotic, reported that exacerbations of psychotic symptoms usually occurred within 7 to 14 days,24 and in some instances could be more severe than symptoms prior to starting clozapine.21,22
Strategies for Preventing Withdrawal-Associated Psychosis
There is an absence of evidence on how clozapine should be discontinued to reduce the risk of withdrawal-associated psychosis as no studies to date have directly compared the effects of different withdrawal regimes. However, a consistent finding is that abrupt clozapine discontinuation is associated with a greater likelihood of withdrawal-associated psychosis.21,22 Consequently, clozapine should be gradually withdrawn unless there is a clinical necessity to stop it abruptly (eg, an immediately life-threatening adverse effect). Hyperbolic discontinuation of antipsychotics has been advocated to reduce the risk of relapse.43 This involves reducing doses by increasingly smaller increments which permits a more linear reduction in receptor blockade.43,44
Patients with treatment-resistant schizophrenia are vulnerable to symptom deterioration.45 Whilst there is evidence to support the superiority of clozapine over other antipsychotics,2,46 there is a paucity of empirical data on which antipsychotic to use after clozapine discontinuation. No head-to-head studies of potential alternative treatments have been published. One randomized clinical trial found that switching from clozapine to zotepine led to a worsening of symptoms compared to clozapine continuation.47 The only randomized placebo-controlled trial found that following abrupt clozapine discontinuation, introducing olanzapine reduced the risk of withdrawal-associated psychosis within 3 to 5 days of discontinuation, compared to placebo.48
There is evidence that switching from clozapine to another antipsychotic that has previously been effective may reduce the risk of withdrawal-associated psychosis.49 A Finnish national registry study found that the lowest risk of psychiatric ward admission and mortality involved clozapine re-initiation, followed by switching to olanzapine.50 Similarly, a Dutch national registry study found that clozapine re-initiation was associated with the lowest risk of treatment failure, followed by switching to olanzapine or risperidone.51 Although olanzapine has the closest molecular structure to clozapine among antipsychotics, open-label studies have consistently found that switching from clozapine to olanzapine leads to an increased risk of relapse, particularly when done abruptly,45,52–55 compared to continuing clozapine. Similar findings have also been observed with risperidone.56
When switching from clozapine to another antipsychotic, the patients’ treatment history may indicate if any previous antipsychotics had been clinically effective. Although clozapine is usually introduced because of a lack of treatment response to other antipsychotics, assessing response is often challenging and can be complicated by sub-therapeutic plasma levels (for example as a result of poor adherence, use of low doses, or rapid metabolism) or illicit substance use.57,58
Studies to date have tended to focus on rapidly switching from clozapine to other antipsychotics, so the role of withdrawal-associated psychotic symptoms cannot be excluded. In the one trial that has attempted a gradual reduction of antipsychotics, patients with first episode psychosis who had their doses gradually reduced, or stopped, showed a higher rate of relapse compared to patients continued on the same dose in the short-term.59 However, in the longer term these patients showed no worsening in symptoms and even exhibited greater social recovery.60 Therefore, it may be feasible for a subset of patients to gradually reduce clozapine and not require an alternative antipsychotic to be commenced. It is important to note, however, that only three of the 128 patients in the trial were prescribed clozapine, and therefore the extent to which findings apply to this medication may be limited.
In summary, clozapine re-initiation is the most effective means of avoiding withdrawal-associated psychosis. Therefore, a key consideration is to review whether clozapine can be safely restarted before considering a switch. If there is evidence that the patient has previously responded, or partially responded to another antipsychotic and it was well-tolerated, this should be considered. Otherwise, an atypical antipsychotic should be selected based on individual patient factors (for example, propensity toward metabolic and other adverse effects). Plasma levels may be particularly informative to ensure therapeutic levels are achieved. A subset of patients gradually discontinued on clozapine may not require an alternative antipsychotic to be commenced, however further research in this area is indicated.
Augmentation Strategies for Preventing Withdrawal-Associated Psychosis
Studies evaluating medications that may be used alongside antipsychotics to reduce the risk of withdrawal-associated psychosis after clozapine discontinuation are scarce, and there are no specific medications licensed for this purpose. However, there is some evidence that serotonin receptor antagonists may be helpful. A small open-label study found that cyproheptadine alongside antipsychotic medication was associated with a lower rate of withdrawal-associated psychosis.24 Another small open-label study found that pimavanserin, which is licensed for use in Parkinson’s disease psychosis, was an effective adjunct to antipsychotic medication in patients with treatment-resistant schizophrenia.61 There is also evidence from a small retrospective study that anticholinergic medication is associated with a reduced likelihood of psychotic relapse in patients who are abruptly discontinued with clozapine.62 Nevertheless, overall evidence that augmenting antipsychotics with serotonin receptor antagonists or anticholinergic medication reduces the risk of withdrawal-associated psychosis is limited (table 2).
Recommendations on the Management of Clozapine Withdrawal-Associated Psychosis
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise the risk of withdrawal-associated psychosis | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| Aim for hyperbolic discontinuation of clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| When stopping electively, the rate of hyperbolic discontinuation should be titrated to the rate at which a patient can tolerate, through shared decision making | Consensus based | - | - |
| If feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If clozapine re-initiation is not feasible, consider restarting an antipsychotic that had previously been effective or partially effective | Evidence-based | (IIb) evidence from at least one other type of quasi-experimental study | (B) directly based on category II evidence |
| In the absence of a history of previous effective antipsychotic treatment, consider switching to an atypical antipsychotic following established guidelines (eg, Maudsley Prescribing Guidelines) and taking patient factors into consideration | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, aim for a gradual cross-taper whilst considering the potential effects of drug interactions | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, consider measuring antipsychotic blood levels to ensure that they are in the therapeutic range | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| Consider augmenting antipsychotics with a serotonin receptor antagonists or anticholinergic medication to reduce the risk of rebound psychosis | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise the risk of withdrawal-associated psychosis | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| Aim for hyperbolic discontinuation of clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| When stopping electively, the rate of hyperbolic discontinuation should be titrated to the rate at which a patient can tolerate, through shared decision making | Consensus based | - | - |
| If feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If clozapine re-initiation is not feasible, consider restarting an antipsychotic that had previously been effective or partially effective | Evidence-based | (IIb) evidence from at least one other type of quasi-experimental study | (B) directly based on category II evidence |
| In the absence of a history of previous effective antipsychotic treatment, consider switching to an atypical antipsychotic following established guidelines (eg, Maudsley Prescribing Guidelines) and taking patient factors into consideration | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, aim for a gradual cross-taper whilst considering the potential effects of drug interactions | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, consider measuring antipsychotic blood levels to ensure that they are in the therapeutic range | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| Consider augmenting antipsychotics with a serotonin receptor antagonists or anticholinergic medication to reduce the risk of rebound psychosis | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
Recommendations on the Management of Clozapine Withdrawal-Associated Psychosis
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise the risk of withdrawal-associated psychosis | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| Aim for hyperbolic discontinuation of clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| When stopping electively, the rate of hyperbolic discontinuation should be titrated to the rate at which a patient can tolerate, through shared decision making | Consensus based | - | - |
| If feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If clozapine re-initiation is not feasible, consider restarting an antipsychotic that had previously been effective or partially effective | Evidence-based | (IIb) evidence from at least one other type of quasi-experimental study | (B) directly based on category II evidence |
| In the absence of a history of previous effective antipsychotic treatment, consider switching to an atypical antipsychotic following established guidelines (eg, Maudsley Prescribing Guidelines) and taking patient factors into consideration | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, aim for a gradual cross-taper whilst considering the potential effects of drug interactions | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, consider measuring antipsychotic blood levels to ensure that they are in the therapeutic range | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| Consider augmenting antipsychotics with a serotonin receptor antagonists or anticholinergic medication to reduce the risk of rebound psychosis | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise the risk of withdrawal-associated psychosis | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| Aim for hyperbolic discontinuation of clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| When stopping electively, the rate of hyperbolic discontinuation should be titrated to the rate at which a patient can tolerate, through shared decision making | Consensus based | - | - |
| If feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If clozapine re-initiation is not feasible, consider restarting an antipsychotic that had previously been effective or partially effective | Evidence-based | (IIb) evidence from at least one other type of quasi-experimental study | (B) directly based on category II evidence |
| In the absence of a history of previous effective antipsychotic treatment, consider switching to an atypical antipsychotic following established guidelines (eg, Maudsley Prescribing Guidelines) and taking patient factors into consideration | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, aim for a gradual cross-taper whilst considering the potential effects of drug interactions | Evidence-based | (Ib) evidence from at least one randomised controlled trial | (B) extrapolated recommendation from category I evidence |
| If switching to another antipsychotic, consider measuring antipsychotic blood levels to ensure that they are in the therapeutic range | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
| Consider augmenting antipsychotics with a serotonin receptor antagonists or anticholinergic medication to reduce the risk of rebound psychosis | Evidence-based | (III) evidence from non-experimental descriptive study | (C) directly based on category III evidence |
Cholinergic Discontinuation Symptoms
Cholinergic symptoms, commonly termed “cholinergic rebound,” 63 have been reported following both the abrupt and gradual withdrawal of clozapine.48,49,64–68 Symptoms have been described following the discontinuation of clozapine at doses as low as 50 mg daily.69 Evidence from a small prospective study suggests that the prevalence of cholinergic discontinuation symptoms following the abrupt withdrawal of clozapine may be as high as 50%.49 In most cases, symptoms emerge within a few days and continue for several weeks.67,70–73 Psychiatric symptoms include agitation, delirium, and hallucinations,65,74,75 while physical symptoms include vomiting, diarrhea, headache, diaphoresis, dystonia, and dyskinesias.65,76
The most likely mechanism for cholinergic rebound is the upregulation of muscarinic acetylcholine receptors by clozapine, leading to super-sensitivity after discontinuation.74,75 Cholinergic rebound is also associated with a range of other central nervous system acting medications with anticholinergic effects, such as tricyclic antidepressants and Parkinson’s disease medications.74,77–82
Acute movement disorders after clozapine discontinuation, in particular extrapyramidal symptoms (EPS) may be related to an acetylcholine-dopamine imbalance in the basal ganglia,70,83–86 although other mechanisms have also been postulated.69 Patients who have experienced EPS with other antipsychotics appear to be at particular risk.68,70,85,87 EPS have been reported within a fortnight of clozapine discontinuation and may co-exist with other symptoms of cholinergic rebound.70,87 Case report evidence suggests that acute movement disorders may be more likely to occur with the simultaneous withdrawal of other anticholinergic agents.83
Strategies to Reduce the Risk of Cholinergic Discontinuation Symptoms
Clozapine-induced cholinergic rebound has not been widely studied, with evidence limited to case reports and case series. For example, it is unclear whether the risk of cholinergic discontinuation symptoms is related to the duration of clozapine treatment. A small case series suggests that patients treated with medication that has anticholinergic properties, such as tricyclic antidepressants and Parkinson’s disease medications, at the time of clozapine discontinuation may be less susceptible to cholinergic rebound.62
There is minimal evidence regarding the optimal regimen for stopping clozapine to avoid cholinergic rebound. Although the current advice is that clozapine should be tapered off over at least 1−2 weeks16,28, there have been cases of cholinergic rebound occurring when patients have been stopped on clozapine flowing this regime65,88; therefore, a more gradual tapering regimen should be considered.27 Furthermore, lower doses of clozapine do not appear to prevent the onset of cholinergic rebound.69 Whilst hyperbolic tapering has been advocated to prevent withdrawal symptoms in other psychiatric medications,43,44,89 it is unclear whether this reduces the risk of clozapine-induced cholinergic rebound. Similarly, the clinical usefulness of prophylactic anticholinergic medication remains unknown.64 Clozapine has a greater affinity to muscarinic receptors than other antipsychotics with anticholinergic properties, such as olanzapine and quetiapine.88,90 This may explain why switching from clozapine to these antipsychotics does not appear to prevent cholinergic discontinuation symptoms.
Strategies to Treat Cholinergic Discontinuation Symptoms
If abrupt discontinuation of clozapine is necessary, the current literature suggests anticholinergic medication may prevent, or reduce the severity of cholinergic rebound. Case reports have found an association between trihexyphenidyl,88 benztropine,91 and biperiden69 initiation and an improvement in clozapine-induced cholinergic rebound symptoms. However, the optimal drug, dose, and duration remains unknown.64,84 When considering anticholinergic medication, it is also important to consider clinical factors such as cholinergic load, addiction vulnerability, and contraindications, such as glaucoma.
Retrospective evidence suggests that re-initiation of clozapine is effective in treating cholinergic rebound.65,92,93 In one case series, re-introducing clozapine at relatively low doses (25–50 mg daily) was sufficient to improve symptoms.65 However, in the absence of studies incorporating a comparison group, it is difficult to evaluate treatment strategies. Furthermore, clozapine re-initiation may not always be feasible (table 3).
Recommendations on the Management of Clozapine-Withdrawal-Induced Cholinergic Rebound
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider an anticholinergic agent to treat cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider an anticholinergic agent to treat cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
Recommendations on the Management of Clozapine-Withdrawal-Induced Cholinergic Rebound
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider an anticholinergic agent to treat cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider an anticholinergic agent to treat cholinergic rebound symptoms | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where feasible, reinitiate clozapine rather than switching to another antipsychotic | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
Clozapine-Withdrawal-Induced Catatonia
Catatonia is a psychomotor disorder characterized by stupor, posturing, and echo phenomena94 and is thought to arise due to hypoactivity of the GABAergic system. Catatonia has been reported in a subset of patients abruptly discontinued on clozapine, and whilst it is thought to occur rarely, its incidence is unknown. It has mainly been observed in patients without a history of catatonia and who have been treated with clozapine for several years, with symptoms emerging within a week of discontinuation.95 Catatonia is not associated with the discontinuation of other antipsychotics, but is observed following benzodiazepine withdrawal. The similarity between clozapine and benzodiazepine discontinuation precipitating catatonia suggests a possible shared pathophysiology mediated via GABA effects.96 While benzodiazepines are known to directly modulate the GABAergic system, prolonged use of clozapine has been shown to indirectly lead to GABA receptor adaption and a reduction in GABAergic effects.95
Strategies to Reduce the Risk and Treat Clozapine-Withdrawal-Induced Catatonia
Clozapine-withdrawal-induced catatonia has not been widely studied, and support for particular treatment strategies is limited to case reports and case series.95 Based on indirect evidence from other clozapine withdrawal-associated symptoms, abrupt discontinuation should be avoided where possible. In situations where catatonia emerges, clozapine re-initiation is associated with the greatest symptomatic improvement. Furthermore, there is case report evidence that benzodiazepines are effective for some patients,97 however the effects appear to be more modest than in other causes of catatonia. Electroconvulsive therapy (ECT) has been shown to be effective in some case of clozapine-withdrawal-induced catatonia, however in all of the reported cases to date, clozapine re-initiation had not been attempted (table 4).98,99
Recommendations on the Management of Clozapine-Withdrawal-Induced Catatonia
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced catatonia | Consensus based | - | - |
| If feasible, re-initiate clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider benzodiazepine as an adjunctive treatment | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where re-initiation of clozapine or use of benzodiazepines is not feasible, consider ECT | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced catatonia | Consensus based | - | - |
| If feasible, re-initiate clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider benzodiazepine as an adjunctive treatment | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where re-initiation of clozapine or use of benzodiazepines is not feasible, consider ECT | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
Recommendations on the Management of Clozapine-Withdrawal-Induced Catatonia
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced catatonia | Consensus based | - | - |
| If feasible, re-initiate clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider benzodiazepine as an adjunctive treatment | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where re-initiation of clozapine or use of benzodiazepines is not feasible, consider ECT | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced catatonia | Consensus based | - | - |
| If feasible, re-initiate clozapine | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Consider benzodiazepine as an adjunctive treatment | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
| Where re-initiation of clozapine or use of benzodiazepines is not feasible, consider ECT | Evidence-based | (III) evidence from non-experimental descriptive study | (C) Directly based on category III evidence |
Serotonergic Discontinuation Symptoms
Serotoninergic discontinuation symptoms such as agitation, diaphoresis, clonus, and hyperreflexia have been reported in a minority of patients following the abrupt100 and gradual discontinuation of clozapine,101 at doses as low as 37.5 mg daily.102 The prevalence of serotonergic discontinuation symptoms is unknown and risk factors for its emergence remain unclear. Case report evidence suggests that clozapine-induced serotonergic symptoms can arise in the presence100,101 or absence of concomitant serotonergic medications.102 Symptoms typically appear within days of clozapine discontinuation.101 The mechanism by which clozapine withdrawal induces these symptoms is not clear. As clozapine is a potent 5-HT2A antagonist, it has been postulated that its removal may lead to serotonin super-sensitivity.101 Serotonin discontinuation symptoms have also been linked to other antipsychotics with 5-HT2A antagonistic properties, such as aripiprazole103 and quetiapine.104
Strategies to Reduce the Risk and Treat Seterotonergic Discontinuation Symptoms
Clozapine-withdrawal-induced serotonin discontinuation symptoms have not been widely studied, and evidence to support treatment strategies is limited to case reports. Based on indirect evidence from other clozapine withdrawal-associated symptoms, abrupt discontinuation should be avoided where possible. In situations where clozapine-withdrawal-induced serotonin discontinuation symptoms emerge, there is case report evidence that symptoms can be reduced through the cessation of all serotonergic medications,101 or use of the anticholinergic and serotonergic antagonist cyproheptadine (table 5).100
Recommendations on the Management of Clozapine-Withdrawal-Induced Serotonergic Discontinuation Symptoms
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced serotonergic discontinuation symptoms | Consensus based | - | - |
| Cease any concomitant serotonergic medications and provide supportive care | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| Consider short-term use of cyproheptadine in moderate and severe cases | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| If feasible, re-initiate clozapine | Consensus based | - | - |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced serotonergic discontinuation symptoms | Consensus based | - | - |
| Cease any concomitant serotonergic medications and provide supportive care | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| Consider short-term use of cyproheptadine in moderate and severe cases | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| If feasible, re-initiate clozapine | Consensus based | - | - |
Recommendations on the Management of Clozapine-Withdrawal-Induced Serotonergic Discontinuation Symptoms
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced serotonergic discontinuation symptoms | Consensus based | - | - |
| Cease any concomitant serotonergic medications and provide supportive care | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| Consider short-term use of cyproheptadine in moderate and severe cases | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| If feasible, re-initiate clozapine | Consensus based | - | - |
| Recommendations . | Type . | Level of Evidence . | Strength of Recommendation . |
|---|---|---|---|
| Avoid abrupt discontinuation of clozapine to minimise risk of clozapine-withdrawal-induced serotonergic discontinuation symptoms | Consensus based | - | - |
| Cease any concomitant serotonergic medications and provide supportive care | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| Consider short-term use of cyproheptadine in moderate and severe cases | Evidence-based | (III) Evidence from non-representative surveys or case reports | (C) Directly based on category III evidence |
| If feasible, re-initiate clozapine | Consensus based | - | - |
Developing a Protocol for the Safe Discontinuation of Clozapine
We explored the feasibility of developing evidence-based recommendations for the safe discontinuation of clozapine in emergency and elective scenarios. A total of 19 provisional recommendations were developed, of which 15 were evidence-based and 4 were consensus-based. The highest level of evidence supporting each recommendation was graded I to IV following established guidelines.40 The most common level of evidence was grade III (ie, non-experimental descriptive studies). The strength of recommendations, based on the grade of evidence and the relevance to the recommendation, were also rated and graded between A and D. The most common strength of recommendation was grade C (ie, directly based on category III evidence or extrapolated recommendation from category I or II evidence). Overall, findings suggest that it is feasible to develop evidence-based recommendations, however the strength of recommendations are limited by a reliance on non-experimental descriptive studies.
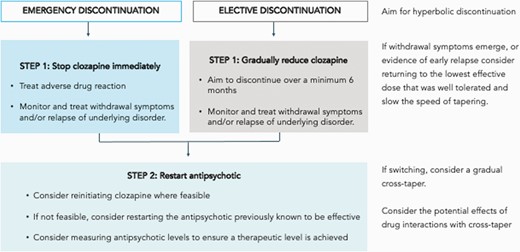
Based on these provisional recommendations, we devised a simple protocol for the discontinuation of clozapine (figure 1). The first step involves determining whether clozapine discontinuation is necessary. For example, in the case of adverse effects, these can often be successfully managed without stopping clozapine. It is also worth considering whether any adverse effects are likely to be transient and exploring whether the patient would be willing to tolerate them in view of the benefits of clozapine treatment. For patients who find regular blood tests challenging, it is worth exploring whether the frequency of blood tests could be reduced, or a capillary sample (using a point of care device) be used in place of a venous blood sample.105
The second step concerns the safe discontinuation of clozapine, when other options are not feasible. In the case of an emergency, clozapine should be stopped immediately and the adverse drug reaction treated in the appropriate medical setting. It is important that physical and mental health be closely monitored and relapse and/or withdrawal symptoms proactively managed
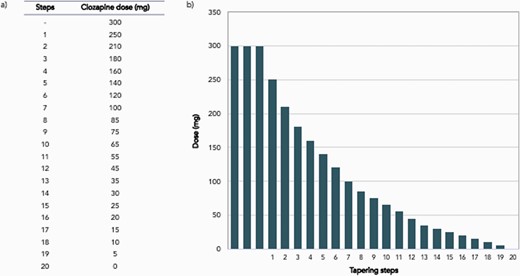
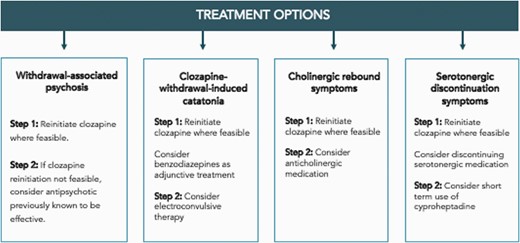
When stopping electively, it is recommended clozapine discontinuation takes place over a minimum of 6 months following a hyperbolic discontinuation regime (figure 2).43 Decisions regarding the planning and implementing of tapering regimes should be made collaboratively with patients. If withdrawal symptoms emerge during the tapering period, or if there is early evidence of relapse, consider returning to the lowest dose that was well-tolerated and reduce the speed of tapering. Strategies to manage specific clozapine discontinuation symptoms are summarized in figure 3.
Example hyperbolic discontinuation regime for a patient treated on stable dose of 300mg of clozapine (adapted from Horowitz et al 2021). Hyperbolic discontinuation involves dose reductions in increasingly smaller increments that approximate linear decreases in receptor occupancy. In this example, each step is equivalent to approximating a 2.5 percentage point reduction in D2 blockade. Following the law of mass effect, a similar relationship can be expected for other receptor types, such as cholinergic and histaminergic. Suggested interval between dose changes are 6−12 weeks, however this can be tailored to the individual. For example, in the case of a patient where withdrawal symptoms are a particular concern, a slower taper can be achieved by increasing the interval between dose reductions and/or by smaller dose reductions. If withdrawal symptoms emerge (which can include psychotic symptoms) consider a longer period on the same dose for stabilisation (which can take weeks or even months), or returning to a higher dose, until symptoms resolve. Thereafter taper more gradually. The emergence of withdrawal symptoms does not necessarily indicate that a patient cannot discontinue clozapine successfully. During discontinuation, it is important to closely monitor other factors that could affect clozapine plasma levels and therefore receptor blockade, such as concomitant medication and smoking status. Hyperbolic discontinuation may lead to the administration of very small doses during the final stages of the taper, which may require splitting tablets or use of liquid formulations.
Provisional recommendations on the management of clozapine discontinuation symptoms. Patients may experience one or more clozapine-withdrawal discontinuation symptoms.
Finally, the third step considers reinitiating antipsychotic treatment. Where feasible, treatment should be clozapine, however in situations where this is not possible, an antipsychotic previously known to be effective and well-tolerated by the patient is recommended. To ensure that a therapeutic level is achieved, measuring antipsychotic plasma levels is advisable (for clozapine as well as non-clozapine antipsychotic medication). If switching to an alternative antipsychotic, a gradual cross-taper should be considered, taking into account potential drug interactions.
Discussion
Studies of treatment-resistant schizophrenia have consistently demonstrated that clozapine is the most effective treatment.75 Sustained use of clozapine induces an array of neuronal adaptions and it remains unclear how long it takes for these changes to be reversed, however existing literature suggests that this takes weeks to months, or longer. This poses a challenge to a clinician when there is a clinical need to discontinue clozapine, whilst ensuring the risk of relapse and withdrawal symptoms is minimized.
We explored the feasibility of developing practical guidance focused on minimizing the risk of relapse and withdrawal symptoms, managing withdrawal phenomena, and commencing alternatives treatment when clozapine is discontinued. A key feature was the recommendation that clozapine should be reduced over at least six months unless the clinical situation necessitates a more urgent cessation. This is substantially longer than has previously been suggested and is motivated by evidence that the likeliness of developing withdrawal symptoms is closely associated with how abruptly clozapine is discontinued. This is likely to be patient dependent and individual factors, such as length of clozapine treatment, are likely to impact on the susceptibility towards developing withdrawal symptoms.
The management of treatment-resistant schizophrenia when clozapine cannot be used remains a major therapeutic challenge.76 The majority of patients with treatment-resistant schizophrenia benefit from being continued on clozapine.106 Epidemiological data suggest that the commonest reason for clozapine discontinuation are adverse drug reactions, many of which can be managed with pharmacological and non-pharmacological interventions.7 In the absence of effective alternatives, it is sensible to attempt to manage adverse effects before clozapine is discontinued. As well as adverse drug reactions, we suggest clinicians counsel patents on the nature and potential risk of withdrawal symptoms before clozapine is initiated. Furthermore, patients should be advised that if stopping clozapine for any reason, this should be done gradually and in collaboration with their clinician.
Limitations
First, although we were able to derive the majority recommendation on existing evidence, there was a reliance on studies of medium to low quality. Second, where possible we based recommendation upon clinical research. However, in some circumstances, this was not possible, and therefore a consensus approach, or non-clinical evidence was utilized. For example, in the absence of clinical studies, recommendations regarding a hyperbolic discontinuation schedule were based upon imaging studies. Third, the recommendations do not account for individual differences. For example, there is likely to be important patient differences in the propensity towards clozapine associated withdrawal symptoms. As a result, a ‘one size fits all’ approach may not suffice, and a clinician is likely to have to tailor a regime to a patient. Fourth, in proposing a hyperbolic discontinuation regime, a linear relationship between receptor occupancy and the likeliness of withdrawal effects is assumed. Whilst there is some empirical support for such a relationship regarding clozapine response,43 this has yet to be confirmed for withdrawal effects. Fifth, in proposing a schedule for the discontinuation of clozapine, we used evidence from imaging studies exploring the effects of clozapine on D2 receptors.107 Clozapine is known to act on a range of receptors including α1, H1, M1, 5-HT2A, 5-HT2B, 5-HT2C, D1, D3, D4, and D5 receptors which may all potentially play a role in withdrawal symptoms.43,108,109 However, the relationship between clozapine dose and receptor occupancy across receptor subtypes can be expected to follow a similar hyperbolic relationship.43
Outstanding Questions
Whilst progress has been made in understanding clozapine-related withdrawal effects, many important questions remain, particularly around epidemiology and treatment (table 6). This highlights the importance of future research being methodologically rigorous, utilizing large samples and well-controlled prospective designs. Further, research is indicated to elucidate the mechanisms and preventative strategies. Finally, clinical evaluation of a proposed guideline (or at the very least, key elements thereof) is an important next step. For example, the effects of hyperbolic discontinuation of clozapine on relapse and withdrawal symptoms should be clinically evaluated using randomized clinical trial methodology against linear tapers. A comparison of different hyperbolic taper regimes would also be informative. In exploring the available evidence and assessing feasibility, it is hoped that this review makes a step towards a clinically useful and evidence-based guideline to minimize the risk of withdrawal symptoms and relapse in patients discontinued on clozapine.
Outstanding Questions in the Epidemiology and Treatment of Clozapine-Withdrawal Symptoms
| Epidemiology |
| • To what extent do clozapine-withdrawal symptoms overlap with relapse? |
| • What factors increase susceptibility to clozapine-withdrawal symptoms? |
| • Is there an association between clozapine dose and risk of withdrawal symptoms? |
| • Is there an association between the duration of clozapine treatment and withdrawal symptoms? |
| • What is the optimal tapering period to reduce the risk of clozapine-withdrawal symptoms? |
| • How long do clozapine-withdrawal symptoms last for? |
| • Is hyperbolic tapering effective in reducing the risk of clozapine-withdrawal symptoms? |
| • What is the mechanism underlying clozapine-withdrawal-induced catatonia? |
| • Are clozapine-withdrawal serotonergic symptoms distinct from serotonin syndrome? |
| Treatment |
| • Is there a role for medications such as serotonin receptor antagonists or anticholinergic medication used prophylactically to reduce the risk of withdrawal symptoms? |
| • Does anticholinergic medication reduce the risk of cholinergic rebound? |
| • How effective are treatments for catatonia and cholinergic and serotonergic symptoms? |
| • Are peripherally-acting anticholinergics more effective than centrally-acting anticholinergics in treating cholinergic rebound? |
| Epidemiology |
| • To what extent do clozapine-withdrawal symptoms overlap with relapse? |
| • What factors increase susceptibility to clozapine-withdrawal symptoms? |
| • Is there an association between clozapine dose and risk of withdrawal symptoms? |
| • Is there an association between the duration of clozapine treatment and withdrawal symptoms? |
| • What is the optimal tapering period to reduce the risk of clozapine-withdrawal symptoms? |
| • How long do clozapine-withdrawal symptoms last for? |
| • Is hyperbolic tapering effective in reducing the risk of clozapine-withdrawal symptoms? |
| • What is the mechanism underlying clozapine-withdrawal-induced catatonia? |
| • Are clozapine-withdrawal serotonergic symptoms distinct from serotonin syndrome? |
| Treatment |
| • Is there a role for medications such as serotonin receptor antagonists or anticholinergic medication used prophylactically to reduce the risk of withdrawal symptoms? |
| • Does anticholinergic medication reduce the risk of cholinergic rebound? |
| • How effective are treatments for catatonia and cholinergic and serotonergic symptoms? |
| • Are peripherally-acting anticholinergics more effective than centrally-acting anticholinergics in treating cholinergic rebound? |
Outstanding Questions in the Epidemiology and Treatment of Clozapine-Withdrawal Symptoms
| Epidemiology |
| • To what extent do clozapine-withdrawal symptoms overlap with relapse? |
| • What factors increase susceptibility to clozapine-withdrawal symptoms? |
| • Is there an association between clozapine dose and risk of withdrawal symptoms? |
| • Is there an association between the duration of clozapine treatment and withdrawal symptoms? |
| • What is the optimal tapering period to reduce the risk of clozapine-withdrawal symptoms? |
| • How long do clozapine-withdrawal symptoms last for? |
| • Is hyperbolic tapering effective in reducing the risk of clozapine-withdrawal symptoms? |
| • What is the mechanism underlying clozapine-withdrawal-induced catatonia? |
| • Are clozapine-withdrawal serotonergic symptoms distinct from serotonin syndrome? |
| Treatment |
| • Is there a role for medications such as serotonin receptor antagonists or anticholinergic medication used prophylactically to reduce the risk of withdrawal symptoms? |
| • Does anticholinergic medication reduce the risk of cholinergic rebound? |
| • How effective are treatments for catatonia and cholinergic and serotonergic symptoms? |
| • Are peripherally-acting anticholinergics more effective than centrally-acting anticholinergics in treating cholinergic rebound? |
| Epidemiology |
| • To what extent do clozapine-withdrawal symptoms overlap with relapse? |
| • What factors increase susceptibility to clozapine-withdrawal symptoms? |
| • Is there an association between clozapine dose and risk of withdrawal symptoms? |
| • Is there an association between the duration of clozapine treatment and withdrawal symptoms? |
| • What is the optimal tapering period to reduce the risk of clozapine-withdrawal symptoms? |
| • How long do clozapine-withdrawal symptoms last for? |
| • Is hyperbolic tapering effective in reducing the risk of clozapine-withdrawal symptoms? |
| • What is the mechanism underlying clozapine-withdrawal-induced catatonia? |
| • Are clozapine-withdrawal serotonergic symptoms distinct from serotonin syndrome? |
| Treatment |
| • Is there a role for medications such as serotonin receptor antagonists or anticholinergic medication used prophylactically to reduce the risk of withdrawal symptoms? |
| • Does anticholinergic medication reduce the risk of cholinergic rebound? |
| • How effective are treatments for catatonia and cholinergic and serotonergic symptoms? |
| • Are peripherally-acting anticholinergics more effective than centrally-acting anticholinergics in treating cholinergic rebound? |
Acknowledgments
We would like to thank the South London and Maudsley (SLaM) NHS Foundation Trust Psychosis Clinical Academic Group for supporting the development of the guideline. We would also like to thank the SLaM Psychosis Clinical Academic Group Service User Advisory Group for reviewing and critically appraising the guideline.
Funding
This work was supported by a grant from the Maudsley Charity.
Conflicts of Interest
D.T. has received personal fees from H. Lundbeck and Janssen unrelated to this study. J.M. has received research funding from H. Lundbeck unrelated to this study. Remaining authors declare no conflict of interest.
References
Author notes
Joint first authors;
Joint senior authors.