-
PDF
- Split View
-
Views
-
Cite
Cite
L Bam, Z M McLaren, E Coetzee, K H von Leipzig, Reducing stock-outs of essential tuberculosis medicines: a system dynamics modelling approach to supply chain management, Health Policy and Planning, Volume 32, Issue 8, October 2017, Pages 1127–1134, https://doi.org/10.1093/heapol/czx057
Close - Share Icon Share
Abstract
The under-performance of supply chains presents a significant hindrance to disease control in developing countries. Stock-outs of essential medicines lead to treatment interruption which can force changes in patient drug regimens, drive drug resistance and increase mortality. This study is one of few to quantitatively evaluate the effectiveness of supply chain policies in reducing shortages and costs. This study develops a systems dynamics simulation model of the downstream supply chain for amikacin, a second-line tuberculosis drug using 10 years of South African data. We evaluate current supply chain performance in terms of reliability, responsiveness and agility, following the widely-used Supply Chain Operation Reference framework. We simulate 141 scenarios that represent different combinations of supplier characteristics, inventory management strategies and demand forecasting methods to identify the Pareto optimal set of management policies that jointly minimize the number of shortages and total cost. Despite long supplier lead times and unpredictable demand, the amikacin supply chain is 98% reliable and agile enough to accommodate a 20% increase in demand without a shortage. However, this is accomplished by overstocking amikacin by 167%, which incurs high holding costs. The responsiveness of suppliers is low: only 57% of orders are delivered to the central provincial drug depot within one month. We identify three Pareto optimal safety stock management policies. Short supplier lead time can produce Pareto optimal outcomes even in the absence of other optimal policies. This study produces concrete, actionable guidelines to cost-effectively reduce stock-outs by implementing optimal supply chain policies. Preferentially selecting drug suppliers with short lead times accommodates unexpected changes in demand. Optimal supply chain management should be an essential component of national policy to reduce the mortality rate.
Key Messages
Our analysis identified two key challenges with the current supply chain for second-line tuberculosis drugs in South Africa—inefficiently high inventory levels, and long lead times for suppliers.
We demonstrate that implementing a safety stock policy that keeps safety stock at a set level can dramatically reduce the number of shortages and reduce costs.
Preferentially selecting drug suppliers with short lead times will improve the efficiency of the entire downstream supply chain by more easily accommodating unexpected changes in demand for tuberculosis drugs.
Introduction
Stock-outs and shortages of essential medicines in developing countries continue to pose a challenge for public health facilities (Institute of Medicine 2013). The resulting treatment interruptions can increase patient infectiousness, drive the development of increasingly more drug resistant forms of disease, and lead to worse patient outcomes. Some of the challenges facing the drug supply chain that links global producers to health facilities via local suppliers and centralized depots include: (i) low demand for drugs due to limited diagnostic capacity or limited purchasing power of patients, (ii) poor demand forecasting mechanisms, (iii) fragmentation of the supply chain into multiple segments subject to different regulatory requirements, (iv) separate financing mechanisms for international and national components of the supply chain that increases uncertainty in the market, (v) short shelf-lives of drugs and (vi) long lead times from order placement to delivery (Institute of Medicine 2013).
The under-performance of the global supply chain for essential medicines was identified by the Institute of Medicine (IOM) as a significant hindrance to global public health efforts worldwide. Because supply chains are complex systems, quantitative modelling is required to determine the impact of potential improvements to supply chains and how these changes contribute to fighting global public health threats. Few studies have modelled supply chains in developing countries, however.
In this study, we employ systems dynamics simulation methods to model the system of suppliers, depots and facilities to generate insight into both operational and strategic decision making in a supply chain (Behdani 2012; Owen et al. 2010). Both simulation modelling in general and system dynamics modelling in particular are widely used in supply chain design and analysis (see Akkermans and Dellaert 2005; Angerhofer and Angelides 2000; Beamon 1998; Campuzano and Mula 2011; Min and Zhou 2002; Terzi and Cavalieri 2004; for reviews); however, a systematic search found only three studies that have used system dynamics for modelling pharmaceutical supply chains (Asamoah et al. 2011; Behzad et al. 2011; Zamora Aguas et al. 2013).
We model the performance of the supply chain for amikacin, a second-line anti-tuberculosis drug (SLD) that is widely used during the first phase of multi-drug resistant tuberculosis (MDR-TB) treatment. In the case of TB, stock-outs of anti-tuberculosis drugs lead to treatment interruption which can force changes in patient drug regimens, drive drug resistance and increase mortality. For example, one study of HIV patients found that stock-outs of anti-retroviral treatment more than doubled the risk of treatment interruption or death (Pasquet et al. 2010). TB recently overtook HIV to become the leading infectious disease cause of death worldwide, causing an estimated 1.5 million deaths in 2015 (World Health Organisation 2015). We use data from the Western Cape province of South Africa (where Cape Town is located), which has a high prevalence of MDR-TB and high-quality data on amikacin use. We are one of the first studies to use system dynamics modelling to examine pharmaceutical supply chains in South Africa.
We analyse the current performance of the supply chain using the Supply Chain Operations Reference (SCOR) criteria of reliability, responsiveness and agility. The SCOR framework is the most widely used framework to model and benchmark dynamic supply chains (Huang et al. 2005; Persson and Araldi 2009). It was developed over the last two decades by the American Production and Inventory Control Society (APICS), the global professional association for supply chain and operations management. Our approach of supply chain performance optimization with analysis tools aids the supply chain to develop from an intermediate “organized” phase to a more advanced “integrated” phase (John Snow, Inc. 2012).
We examine the expected impact of reduced supplier lead time and improved reliability as well as potential changes to inventory management and provincial demand forecasting practices on (i) the number of stock-outs that occur and (ii) the total costs including stock holding and obsolescence costs as well as the cost of replacement therapy (in the event of a stock-out). Our findings demonstrate that the amikacin supply chain exhibits sub-optimal inventory management. We identify a set of straightforward, actionable changes in the approach to inventory management that can effectively reduce stock-outs of SLDs while minimizing costs, thereby saving lives and reducing health care expenditures.
Background
There is general agreement that under-performing supply chains contribute to high prices and limited availability of quality assured SLDs, which hinder effective control of TB and MDR-TB (Institute of Medicine 2012, 2013). MDR-TB has become a public health emergency across the globe, with the BRICS (Brazil, Russia, India, China and South Africa) countries carrying the burden of almost 60% of all notified MDR-TB cases (Médecins Sans Frontières 2013). MDR-TB is resistant to both key first line drugs, rifampicin and isoniazid, and requires a minimum of 18 months of therapy with SLDs, which are more expensive, less potent and more toxic than first-line drugs (Institute of Medicine 2013). MDR-TB can be acquired through incorrect or incomplete treatment of drug-susceptible TB or be transmitted by airborne droplets when someone with active MDR-TB coughs, sneezes or speaks. MDR-TB is fatal if left untreated, but it can be cured with an 18–24-month course of anti-tuberculosis therapy (Institute of Medicine 2013).
Optimal management and operation of the downstream supply chain involves addressing four specific components—public tendering, demand forecasting, inventory management and distribution—to reduce stock-outs and minimize costs. A number of recent studies have identified inefficiencies in supply chain management in developing countries and proposed solutions based on changes in operational practices. Quick (1982) suggested the implementation of classic inventory models and closer supplier monitoring to reduce variability in the drug procurement process. Jahre et al. (2012) posited that reducing lead times and increasing order frequencies could allow safety stocks to be reduced while preventing understocking. Jatau et al. (2015) stressed early warning measures and easy to implement inventory policies to improve efficiency. Procurement inefficiencies, unreliable distribution systems and shortages of skilled supply chain practitioners were identified as contributors to poor functioning of supply chains in Nigeria and Malawi (Leung et al. 2016; Schouten et al. 2011). Bateman (2013) documented the challenges South Africa faces due to poorly integrated information systems and widespread corruption.
Many components of the supply chain for SLDs in South Africa have the potential to be optimized using changes in operational practices. SLDs are procured on a competitive basis through a public tendering process, which selects suppliers based on specific criteria and sets service level agreements to contract their performance (Department of Health 2016). The outcomes of the tendering process determine supplier lead time and reliability. Inventory management practices are employed to address the challenge of fluctuating demand for SLDs, which may be exacerbated when upstream challenges limit both the number of suppliers and the availability of SLDs (Institute of Medicine 2013). Optimal methods for determining safety stock levels can reduce the occurrence of shortages and minimize holding costs. Accurate demand forecasting methods match supply with demand in order to eliminate lag time, allow funders to plan purchases and allocate resources efficiently (Institute of Medicine 2009). It also reduces uncertainty about future market potential which improves the reliability of drug supply by encouraging the development of new drugs (Institute of Medicine 2013).
Methods
In order to evaluate the expected impact of potential changes in supply chain management and develop solutions for challenges faced by the South African supply chain, we design a system dynamics model of the SLD supply chain in the Western Cape province, which includes the city of Cape Town. We use data from 2004 to 2014 supplied by the central provincial drug depot (Cape Medical Depot or CMD) for (i) orders placed to and received from all CMD suppliers; and (ii) orders placed to and received from the CMD by the 345 patient-serving public healthcare facilities in the Western Cape that it serves.
A system dynamics approach was used in this study because it enables the modelling of complex real-world problems with relative ease of implementation. In contrast, analytical modelling methods (e.g. Graves and Willems 2000) can be mathematically complex to model and solve and therefore tend to require significant simplification of the problem (Campuzano and Mula 2011; Kersten and Saeed 2014). The majority of publications modelling more than one SCOR process use either a discrete event or a system dynamics simulation approach, either of which would be appropriate in this case (Kersten and Saeed 2014). In addition, Besiou et al. (2011) concur that the systems dynamics approach is able to “accurately represent the dynamic complexity of humanitarian operations”.
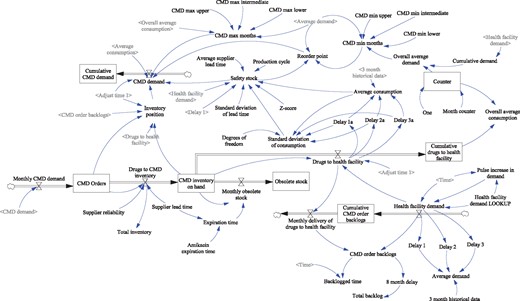
We develop and validate a dynamic model in the Vensim software package based on the causal loop diagram (CLD) depicted in Figure 1, using the data from the Western Cape province with model parameters set for amikacin. The system is modelled in continuous time with feedback loops driving the dynamic behaviour. In the first phase of analysis, the model is used to evaluate the current performance of the downstream supply chain in terms of three SCOR attributes: reliability (defined as the percent of orders that are delivered in full), responsiveness (defined as the average time between when an order is placed and when it is either delivered to or dispatched by the CMD) and agility (defined as the ability to withstand unplanned changes in external variables without causing a shortage) (Mohammadi and Mukhtar 2013).
Dynamic supply chain model of second line tuberculosis drugs in the Western Cape Province, South Africa
In the second phase of analysis, we examine how potential operational changes to the management of the supply chain are likely to affect two performance attributes: (i) the number of shortages; and (ii) the total costs incurred by stock holding, stock obsolescence and replacement therapy. We evaluate potential operational changes to: sales and operations planning to inform public tendering (operationalized through changes to the supplier lead time and reliability); inventory management (operationalized through changes to desired stock levels and safety stock policies); and demand forecasting (operationalized through changes to reorder frequency and reorder quantity). Further details on these potential operational changes can be found in the Methodological Appendix.
First, we evaluate the impact of each potential operational change in isolation while all other changes are at baseline levels and then we evaluate combined scenarios which are formed by adjusting a maximum of two operational variables at a time. We omit the minimum and maximum lead time options from the combined scenarios as it is unlikely that either of these scenarios would be consistently achieved in a real-world setting. This yields an exhaustive list of 20 scenarios in which only one variable or management method is adjusted at a time plus all 121 possible scenarios involving the adjustment of two variables. Note that combinations of the operational variables that cannot be adjusted simultaneously are excluded. All 141 scenarios were evaluated using the system dynamics model to determine which ones are best able to jointly minimize shortages and total costs.
Results
Current performance analysis
We find that the Western Cape province supply chain for amikacin is highly reliable, which is defined as the percent of orders that are delivered in full. Orders delivered from suppliers to the Western Cape CMD were 98% reliable on average over the past 10 years, and orders delivered from the CMD to health facilities were 92% reliable. On the other hand, responsiveness, which is defined as the average time between when an order is placed and when it is either delivered to or dispatched by the CMD, is slow for orders delivered from suppliers to the CMD. Thirty percent of orders were delivered within 2 weeks and 57% of orders were delivered within 1 month; however, 13% of orders took longer than 3 months to be fulfilled. The CMD dispatched 76% of all orders within 48 h of receipt over the 10-year period. This is close to the target of 80% of all orders filled in three working days set in the 2014–15 financial year (Western Cape Department of Health 2014). Viewed in conjunction with the CMD’s general achievement of 94.9% of all orders filled within 72 h in 2014–15, it is a strong indication of the CMD’s high performance in terms of responsiveness (Western Cape Department of Health 2014).
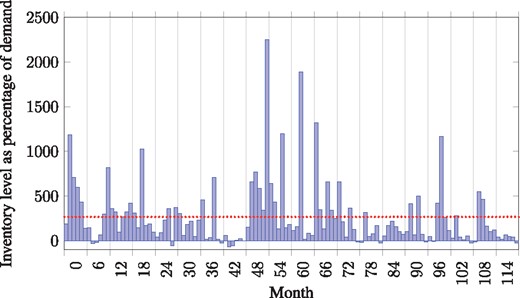
Supply chain agility is defined as the ability to withstand unplanned changes in external variables without causing a shortage. The supply chain was highly agile in that a 20% increase in demand could be satisfied immediately, despite the combination of long supplier lead times and unpredictable demand. Figure 2 shows the inventory levels as a percentage of demand for each month in the analysis. The agility of the supply chain was accomplished by overstocking, with an average of 267% as many units of amikacin being held in inventory than what is required each month. This is a common safety stock practice to ensure agility (Rushton et al. 2014); however, there are holding costs to carrying such a large overstock.
Monthly inventory holding level of amikacin on hand at Cape Medical Depot as a percentage of demand for amikacin. Dotted line indicates the average inventory level of 267% of monthly demand
Scenario modelling
Based on this analysis, enhancing supply chain performance should focus on improving responsiveness, especially from suppliers to the CMD, by (i) effective inventory management; and (ii) sales and operation planning (Huang et al. 2005; Persson and Araldi 2009). Although the SLD supply chain is shown to be agile, the inventory management practices reduce the overstock rate as well as improve responsiveness.
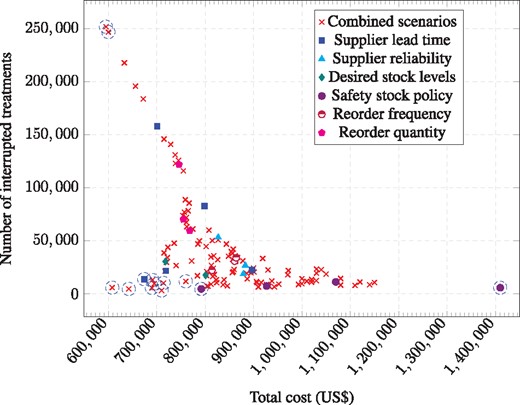
As described in the “Methods” section, our analysis compared the number of shortages and total cost for 141 scenarios representing one or two potential changes each to supplier lead time; supplier reliability; desired stock levels; safety stock; reorder frequency; and/or reorder quantity. Figure 3 shows a scatter plot with each point representing results from one of the 141 scenarios in terms of number of shortages (vertical axis) and total associated cost (horizontal axis). Different marker styles show which variable is adjusted or denotes a combined scenario where two variables were adjusted. Due to competing objectives in our optimization problem: (i) reducing the number of shortages and (ii) minimizing total cost, we identify the Pareto optimal set of outcomes from all scenarios in Figure 3 in which the number of stock-outs cannot be reduced without increasing cost or the cost cannot be reduced without increasing the number of shortages. The Pareto set as well as those that are close to the Pareto frontier are circled in Figure 3 and details of the circled scenarios are given in Table 1.
Results from Pareto optimal and near-Pareto optimal scenarios
| Pareto optimal . | Variables . | Shortagesa . | Total costsa . | |||||
|---|---|---|---|---|---|---|---|---|
| Supplier lead time (months) . | Supplier reliability . | Desired stock levels (months) . | Safety stock policies . | Reorder frequency (monthly frequency) . | Reorder quantity (exponential smoothing) . | |||
| √ | 3 & 5 | %increaseb | 3130 | $710 000 | ||||
| #### | %increaseb | 4470 | $792 000 | |||||
| √ | 0.5 | %increaseb | 4560 | $642 000 | ||||
| %increaseb | 4610 | $792 000 | ||||||
| %increaseb | 1 | 5560 | $690 000 | |||||
| SDc | 5700 | $1410 000 | ||||||
| √ | 2 & 4 | %increaseb | 5840 | $608 000 | ||||
| %increaseb | Alpha = 0.9 | 9590 | $695 000 | |||||
| %increaseb | Alpha = 0.5 | 10 100 | $714 000 | |||||
| 0.5 | SFd | 11,700 | $760 000 | |||||
| 0.5 | 2 & 4 | 13,000 | $691 000 | |||||
| 0.25 | 13,800 | $674 000 | ||||||
| √ | 2 | Alpha = 0.1 | 247,000 | $600 000 | ||||
| √ | 3 | Alpha = 0.1 | 252,000 | $595 000 | ||||
| Pareto optimal . | Variables . | Shortagesa . | Total costsa . | |||||
|---|---|---|---|---|---|---|---|---|
| Supplier lead time (months) . | Supplier reliability . | Desired stock levels (months) . | Safety stock policies . | Reorder frequency (monthly frequency) . | Reorder quantity (exponential smoothing) . | |||
| √ | 3 & 5 | %increaseb | 3130 | $710 000 | ||||
| #### | %increaseb | 4470 | $792 000 | |||||
| √ | 0.5 | %increaseb | 4560 | $642 000 | ||||
| %increaseb | 4610 | $792 000 | ||||||
| %increaseb | 1 | 5560 | $690 000 | |||||
| SDc | 5700 | $1410 000 | ||||||
| √ | 2 & 4 | %increaseb | 5840 | $608 000 | ||||
| %increaseb | Alpha = 0.9 | 9590 | $695 000 | |||||
| %increaseb | Alpha = 0.5 | 10 100 | $714 000 | |||||
| 0.5 | SFd | 11,700 | $760 000 | |||||
| 0.5 | 2 & 4 | 13,000 | $691 000 | |||||
| 0.25 | 13,800 | $674 000 | ||||||
| √ | 2 | Alpha = 0.1 | 247,000 | $600 000 | ||||
| √ | 3 | Alpha = 0.1 | 252,000 | $595 000 | ||||
The table presents characteristics of scenarios circled in Figure 3. Scenarios are ordered from fewest to greatest number of shortages.
To put these amounts into perspective, the baseline simulation model, assuming the current management practices, results in 19 200 shortages at a cost of $880 000.
Safety stock calculated based on % increase in demand.
Safety stock calculated with standard deviation formula.
Safety stock calculated with a safety factor (SF).
Results from Pareto optimal and near-Pareto optimal scenarios
| Pareto optimal . | Variables . | Shortagesa . | Total costsa . | |||||
|---|---|---|---|---|---|---|---|---|
| Supplier lead time (months) . | Supplier reliability . | Desired stock levels (months) . | Safety stock policies . | Reorder frequency (monthly frequency) . | Reorder quantity (exponential smoothing) . | |||
| √ | 3 & 5 | %increaseb | 3130 | $710 000 | ||||
| #### | %increaseb | 4470 | $792 000 | |||||
| √ | 0.5 | %increaseb | 4560 | $642 000 | ||||
| %increaseb | 4610 | $792 000 | ||||||
| %increaseb | 1 | 5560 | $690 000 | |||||
| SDc | 5700 | $1410 000 | ||||||
| √ | 2 & 4 | %increaseb | 5840 | $608 000 | ||||
| %increaseb | Alpha = 0.9 | 9590 | $695 000 | |||||
| %increaseb | Alpha = 0.5 | 10 100 | $714 000 | |||||
| 0.5 | SFd | 11,700 | $760 000 | |||||
| 0.5 | 2 & 4 | 13,000 | $691 000 | |||||
| 0.25 | 13,800 | $674 000 | ||||||
| √ | 2 | Alpha = 0.1 | 247,000 | $600 000 | ||||
| √ | 3 | Alpha = 0.1 | 252,000 | $595 000 | ||||
| Pareto optimal . | Variables . | Shortagesa . | Total costsa . | |||||
|---|---|---|---|---|---|---|---|---|
| Supplier lead time (months) . | Supplier reliability . | Desired stock levels (months) . | Safety stock policies . | Reorder frequency (monthly frequency) . | Reorder quantity (exponential smoothing) . | |||
| √ | 3 & 5 | %increaseb | 3130 | $710 000 | ||||
| #### | %increaseb | 4470 | $792 000 | |||||
| √ | 0.5 | %increaseb | 4560 | $642 000 | ||||
| %increaseb | 4610 | $792 000 | ||||||
| %increaseb | 1 | 5560 | $690 000 | |||||
| SDc | 5700 | $1410 000 | ||||||
| √ | 2 & 4 | %increaseb | 5840 | $608 000 | ||||
| %increaseb | Alpha = 0.9 | 9590 | $695 000 | |||||
| %increaseb | Alpha = 0.5 | 10 100 | $714 000 | |||||
| 0.5 | SFd | 11,700 | $760 000 | |||||
| 0.5 | 2 & 4 | 13,000 | $691 000 | |||||
| 0.25 | 13,800 | $674 000 | ||||||
| √ | 2 | Alpha = 0.1 | 247,000 | $600 000 | ||||
| √ | 3 | Alpha = 0.1 | 252,000 | $595 000 | ||||
The table presents characteristics of scenarios circled in Figure 3. Scenarios are ordered from fewest to greatest number of shortages.
To put these amounts into perspective, the baseline simulation model, assuming the current management practices, results in 19 200 shortages at a cost of $880 000.
Safety stock calculated based on % increase in demand.
Safety stock calculated with standard deviation formula.
Safety stock calculated with a safety factor (SF).
Scatter plot of scenario modelling results for number of shortages (vertical axis) and total associated cost (horizontal axis). Each point represents one of the 141 scenarios. Marker styles show which variable is adjusted or denotes a combined scenario where two variables were adjusted. The set of Pareto optimal and near-Pareto optimal outcomes are circled
Scenarios in Table 1 are ordered from fewest to greatest number of shortages with Pareto optimal scenarios identified in the first column (column 1). Various approaches to calculating safety stock levels (column 5) are cost-saving mechanisms for reducing stock-outs, when used either in isolation or in combination with specific approaches to periodic purchasing (column 6) or demand forecasting to determine the reorder quantity (column 7). Combining the percentage increase approach to calculating safety stock (column 5) with either a regulated approach to calculating desired stock levels (column 4) or a reduction in supplier lead time (column 3), generates Pareto optimal outcomes in terms of the cost-effectiveness of the reduction in stock-outs. The results in Table 1 also highlight the impact of supplier lead time (column 2) on the performance of the supply chain (specifically in the scenario reported in the third row from the bottom). For completeness, two Pareto optimal scenarios that result in the lowest costs but high levels of stock-outs are also reported (bottom two rows). These scenarios resulted when periodic purchasing approaches (column 6) were combined with a specific exponential smoothing approach to demand forecasting (column 7).
Discussion
Our analysis of the current supply chain for the SLD amikacin for TB in South Africa shows that the CMD holds excess levels of inventory following a spike in health facility demand due to the fact that the desired levels of stock are adjusted to reflect historical demand. The demand is however too dynamic for this to be an optimal decision strategy. Three of the optimal policy solutions in Table 1 show that keeping desired levels of stock constant reduces both the costs and the number of shortages at the CMD. The scenario modelling results demonstrate that ordering safety stock when demand fluctuates instead of altering the minimum and maximum desired levels of stock is optimal.
It should be noted that our results on overstocking apply to the CMD since operations (and inventory levels) at the health facility are outside of the scope of this research. However, with 95% of all orders filled from the CMD within 72 h under the current parameters and enrolment based treatment that minimizes demand uncertainty, patient serving health facilities would not need to keep large inventories of safety stock. Keeping safety stock at the CMD rather than in health facilities is likely optimal to preserve the ability to direct safety stock to facilities when and where it is needed as well as the limited storage space at health facilities.
This study shows that the downstream SLD supply chain experiences the prominent problem of long and fluctuating supplier lead times, which can result in stock arriving too late to satisfy sudden spikes in demand and further create an unnecessary surplus in stock following a demand spike. Our results show that, all else equal, selecting suppliers with shorter and less variable lead times improves the performance of the supply chain. The small set of suppliers included in this study represents a range of lead times so it should be possible to distinguish between lead time performance of various suppliers when making procurement decisions. In some cases, a cost increase associated with lower lead time would be infeasible with respect to the health budget, but governments may be able to exert negotiating power to incentivize lower lead times among existing suppliers.
Recent developments in TB policy indicate that challenges to the SLD supply chain are rising, which increases the importance of optimal management of supply chains. Starting in 2011, South Africa rolled out the GeneXpert MTB/RIF testing technology which has significantly increased MDR-TB diagnoses (Meyer-Rath et al. 2012). As these patients are initiated on MDR-TB treatment, the demand for SLDs increases, placing an added burden on the supply chain. McLaren and Burger (2016, unpublished manuscript) found that almost 25% of MDR-TB cases were undiagnosed prior to GeneXpert, so the demand for SLDs will continue to rise. Poor adherence to treatment regimens and a lack of transmission control exacerbates the spread of MDR-TB (Institute of Medicine 2009). On the other hand, improved diagnosis of MDR-TB and more accurate surveillance data will make it easier to forecast demand for SLDs, which could ease some of the supply chain challenges identified in this study. It may also lead to more suppliers entering the MDR-TB drug market which will make the market more competitive and thereby improve supplier performance (Keshavjee and Seung 2008). As resistance to amikacin rises and new drugs are developed and trialled, there will be less reliance on amikacin for MDR-TB treatment, however similar supply chain management challenges will remain for other drugs.
Though our modelling method is based on real-world data, the main limitation is that it is unable to capture all the idiosyncrasies in the supply chain, for example, when pharmacists override protocol in response to a potential stock-out. In addition, no data are available to determine the time lag between shortages experienced at the CMD and each of the 345 health facilities it supplies.
It is important to note that the measure of shortages used in this study is based on instances when insufficient stock is available at the CMD to fulfil the orders made by the health facility, and does not capture shortages that occur at the health facility, for example if they fail to order the correct quantity at the appropriate time. There is insufficient data available to assess health facility supply chain practices.
Our model captures all the costs associated with stock-outs when patients are able to switch seamlessly from one drug to another during a stock-out. However, if stock-outs lead to treatment interruptions then additional costs in terms of increased TB transmission, development of additional drug resistance, patient loss-to-follow-up and death must be considered. Optimal supply chain management is an essential component of reducing the number of deaths due to TB.
Our results apply not only to the amikacin supply chain but also to other drugs since the features of the downstream supply chain are generally not drug-specific, however it would be useful to model the dynamics of other drugs to determine whether a drug-specific supply chain management policy is required. Future work could also examine the impact of changes to the upstream supply chain on downstream supply chain dynamics.
Conclusion
Our analysis identified two key challenges with the current supply chain—inefficiently high inventory levels, and long lead times for suppliers—and simple, specific changes to supply chain management that can dramatically reduce the number of shortages and reduce costs from the current policy that leads to an estimated 19 200 shortages and incurs an estimated cost of $880 000. Implementing a safety stock policy that keeps safety stock at a set level can help avoid stock-outs without the excessively high holding costs. All else equal, preferentially selecting drug suppliers with short lead times will enable the entire downstream supply chain to function more efficiently because any unexpected changes in demand for drugs from the CMD and health facilities can more easily be accommodated. Stakeholders involved in the tender process should not only consider the price per unit of drugs from each potential supplier, but also the costs that would be incurred if the supply chain must compensate for longer supplier lead times. In addition, the solutions we propose for the amikacin supply chain in the Western Cape province are likely to also benefit other South African provinces, other TB drugs and other diseases.
It is not possible to accurately estimate the cost of shortages in terms of lives lost because there is too little data on parameters to inform the calculation. However, the Pareto optimal scenario with the fewest shortages (3130) only costs ∼$100 000 more than the Pareto optimal scenario with the lowest costs (5840 shortages). It is therefore safe to say that if eliminating the difference of 2710 shortages saves at least 15 lives it would be considered “very cost-effective” by World Health Organization standards based on South Africa’s per capita GDP (World Health Organization 2016).
The recent increase in MDR-TB cases diagnosed with GeneXpert technology and initiated on treatment strains the supply chain and raises the risk of MDR-TB drug shortages. Improved supply chain management is therefore a critical component of national TB policy, and greater resources should be allocated towards implementing the recommendations in this study.
Acknowledgments
This research was partially presented in poster format at the World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease in October 2016.
Conflict of interest statement. None declared.
References
Médecins Sans Frontières/International Union Against Tuberculosis and Lung Disease.
Methodological Appendix
Shortages occur when the inventory on hand is unable to satisfy the total demand from health facilities for a specific drug and orders are therefore backlogged. Holding costs are calculated as 25% of the value of the inventory on hand every month. Inventory carrying costs are difficult to calculate accurately, but are assumed to be between 20 and 55%, with a figure of 25% used if no accurate figures are available (Toomey 2000). Obsolescence costs are the value of unused drugs that expire during the period. Shortage costs include the cost of allocating substitute drugs for the remainder of the patient’s 8-month intensive phase of treatment plus the cost of patients developing resistance to amikacin due to treatment interruption or incomplete treatment. Because amikacin stock-outs entail a significant risk of developing resistance not only to amikacin, but also to kanamycin, another SLD with a similar chemical composition, we take a conservative approach and model a switch to capreomycin in the event of a stock-out. The 2011 update of the WHO guideline for the programmatic management of drug-resistant TB states that amikacin and kanamycin can be used interchangeably and that capreomycin is effective in the case of resistance to kanamycin (World Health Organization 2011). The pricing fluctuates, but capreomycin is approximately 3–12 times more expensive than amikacin (Médecins Sans Frontières 2013). In the month of the shortage, all MDR-TB patients will need to be switched from amikacin to the appropriate substitute (in our model, capreomycin); however, patients initiated on MDR-TB treatment 1 or more months after the shortage can be initiated on amikacin.
We use the following potential new supply chain management policies to evaluate which combinations were best able to jointly minimize shortages and total costs.
Supplier lead time
We evaluated lead times obtained from the data over the past 10 years: minimum (0.25 months); 25th percentile (0.5 months); average (1.2 months); 75th percentile (1.6 months) and maximum (5.6 months) to fulfil an order.
Supplier reliability
Reliability, which is the likelihood of receiving the correct quantity of amikacin based on the orders received from the CMD, was set to 50, 80 or 100%.
Desired stock levels
The desired minimum and maximum stock level in inventory is set by the pharmacist, but is often changed due to fluctuations in demand. There is no standard method for choosing these levels, so we used the average level from the data in our modelling. The maximum level is equal to the minimum level of stock plus the time between an order to a supplier and the next scheduled order (i.e. the procurement period).
Safety stock policies
When both the consumption and lead times are highly variable, safety stock levels should be set as a function of the variance (Management Sciences for Health 2012). The production cycle is 1 month because that is the frequency at which safety stock is evaluated.
Reorder frequency
Currently, the CMD reorder frequency is based on a perpetual purchasing approach where orders are placed when stock runs low. We evaluated a periodic purchasing approach where orders are placed at regular scheduled intervals (i.e. procurement periods) by adjusting the calculation for CMD demand for placing orders every 1, 2 or 3 months.
Reorder quantity